Effectiveness of the Stand More AT (SMArT) Work intervention: cluster randomised controlled trial
- PMID: 30305278
- PMCID: PMC6174726
- DOI: 10.1136/bmj.k3870
Effectiveness of the Stand More AT (SMArT) Work intervention: cluster randomised controlled trial
Abstract
Objectives: To evaluate the impact of a multicomponent intervention (Stand More AT (SMArT) Work) designed to reduce sitting time on short (three months), medium (six months), and longer term (12 months) changes in occupational, daily, and prolonged sitting, standing, and physical activity, and physical, psychological, and work related health.
Design: Cluster two arm randomised controlled trial.
Setting: National Health Service trust, England.
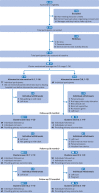
Participants: 37 office clusters (146 participants) of desk based workers: 19 clusters (77 participants) were randomised to the intervention and 18 (69 participants) to control.
Interventions: The intervention group received a height adjustable workstation, a brief seminar with supporting leaflet, workstation instructions with sitting and standing targets, feedback on sitting and physical activity at three time points, posters, action planning and goal setting booklet, self monitoring and prompt tool, and coaching sessions (month 1 and every three months thereafter). The control group continued with usual practice.
Main outcome measures: The primary outcome was occupational sitting time (thigh worn accelerometer). Secondary outcomes were objectively measured daily sitting, prolonged sitting (≥30 minutes), and standing time, physical activity, musculoskeletal problems, self reported work related health (job performance, job satisfaction, work engagement, occupational fatigue, sickness presenteeism, and sickness absenteeism), cognitive function, and self reported psychological measures (mood and affective states, quality of life) assessed at 3, 6, and 12 months. Data were analysed using generalised estimating equation models, accounting for clustering.
Results: A significant difference between groups (in favour of the intervention group) was found in occupational sitting time at 12 months (-83.28 min/workday, 95% confidence interval -116.57 to -49.98, P=0.001). Differences between groups (in favour of the intervention group compared with control) were observed for occupational sitting time at three months (-50.62 min/workday, -78.71 to -22.54, P<0.001) and six months (-64.40 min/workday, -97.31 to -31.50, P<0.001) and daily sitting time at six months (-59.32 min/day, -88.40 to -30.25, P<0.001) and 12 months (-82.39 min/day, -114.54 to -50.26, P=0.001). Group differences (in favour of the intervention group compared with control) were found for prolonged sitting time, standing time, job performance, work engagement, occupational fatigue, sickness presenteeism, daily anxiety, and quality of life. No differences were seen for sickness absenteeism.
Conclusions: SMArT Work successfully reduced sitting time over the short, medium, and longer term, and positive changes were observed in work related and psychological health.
Trial registration: Current Controlled Trials ISRCTN10967042.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work (other than the Department of Health noted in the acknowledgments section); no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. DWD reports grants from National Health and Medical Research Council (Australia), grants from Victorian Health Promotion Foundation (VicHealth), during the conduct of the study. MJD reports personal fees from Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp and Dohme, Boehringer Ingelheim, AstraZeneca, Janssen, Servier, Mitsubishi Tanabe Pharma, and Takeda Pharmaceuticals International, and grants from Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim, and Janssen, outside the submitted work.
Figures


Comment in
-
Reducing sedentary behaviour in the workplace.BMJ. 2018 Oct 10;363:k4061. doi: 10.1136/bmj.k4061. BMJ. 2018. PMID: 30305275 No abstract available.
