Lats1/2 inactivation reveals Hippo function in alveolar type I cell differentiation during lung transition to air breathing
- PMID: 30305289
- PMCID: PMC6240317
- DOI: 10.1242/dev.163105
Lats1/2 inactivation reveals Hippo function in alveolar type I cell differentiation during lung transition to air breathing
Abstract
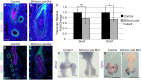
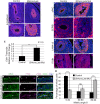
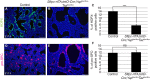
Lung growth to its optimal size at birth is driven by reiterative airway branching followed by differentiation and expansion of alveolar cell types. How this elaborate growth is coordinated with the constraint of the chest is poorly understood. Here, we investigate the role of Hippo signaling, a cardinal pathway in organ size control, in mouse lung development. Unexpectedly, we found that epithelial loss of the Hippo kinase genes Lats1 and Lats2 (Lats1/2) leads to a striking reduction of lung size owing to an early arrest of branching morphogenesis. This growth defect is accompanied by abnormalities in epithelial cell polarity, cell division plane and extracellular matrix deposition, as well as precocious and increased expression of markers for type 1 alveolar epithelial cells (AEC1s), an indicator of terminal differentiation. Increased AEC1s were also observed in transgenic mice with overexpression of a constitutive nuclear form of downstream transcriptional effector YAP. Conversely, loss of Yap and Taz led to decreased AEC1s, demonstrating that the canonical Hippo signaling pathway is both sufficient and necessary to drive AEC1 fate. These findings together reveal unique roles of Hippo-LATS-YAP signaling in the developing mouse lung.
Keywords: Alveolar; Branching; Development; Hippo; Lung; Mouse; Polarity.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

