Dicer-2 Regulates Resistance and Maintains Homeostasis against Zika Virus Infection in Drosophila
- PMID: 30305326
- PMCID: PMC6219897
- DOI: 10.4049/jimmunol.1800597
Dicer-2 Regulates Resistance and Maintains Homeostasis against Zika Virus Infection in Drosophila
Abstract
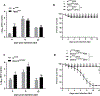
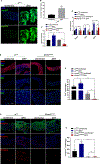
Zika virus (ZIKV) outbreaks pose a massive public health threat in several countries. We have developed an in vivo model to investigate the host-ZIKV interaction in Drosophila We have found that a strain of ZIKV replicates in wild-type flies without reducing their survival ability. We have shown that ZIKV infection triggers RNA interference and that mutating Dicer-2 results in enhanced ZIKV load and increased susceptibility to ZIKV infection. Using a flavivirus-specific Ab, we have found that ZIKV is localized in the gut and fat body cells of the infected wild-type flies and results in their perturbed homeostasis. In addition, Dicer-2 mutants display severely reduced insulin activity, which could contribute toward the increased mortality of these flies. Our work establishes the suitability of Drosophila as the model system to study host-ZIKV dynamics, which is expected to greatly advance our understanding of the molecular and physiological processes that determine the outcome of this disease.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

