The phylogenomics of evolving virus virulence
- PMID: 30305704
- PMCID: PMC7096893
- DOI: 10.1038/s41576-018-0055-5
The phylogenomics of evolving virus virulence
Abstract
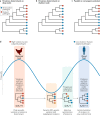
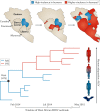
How virulence evolves after a virus jumps to a new host species is central to disease emergence. Our current understanding of virulence evolution is based on insights drawn from two perspectives that have developed largely independently: long-standing evolutionary theory based on limited real data examples that often lack a genomic basis, and experimental studies of virulence-determining mutations using cell culture or animal models. A more comprehensive understanding of virulence mutations and their evolution can be achieved by bridging the gap between these two research pathways through the phylogenomic analysis of virus genome sequence data as a guide to experimental study.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Power RA, Parkhill J, de Oliveira T. Microbial genome-wide association studies: lessons from human GWAS. Nat. Rev. Genet. 2017;18:41–50. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

