Ultra-wide optical coherence tomography angiography in diabetic retinopathy
- PMID: 30306055
- PMCID: PMC6177371
- DOI: 10.21037/qims.2018.09.02
Ultra-wide optical coherence tomography angiography in diabetic retinopathy
Abstract
Background: To implement an ultra-wide optical coherence tomography angiography imaging (UW-OCTA) modality in eyes with diabetic retinopathy (DR) with the aim of quantifying the burden of microvascular disease at baseline and subsequent clinic visits.
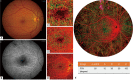
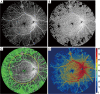
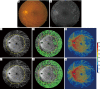
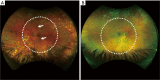
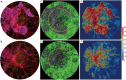
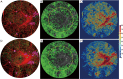
Methods: UW-OCTA was implemented on a 1,060 nm swept source (SS) OCTA engine running at 100 kHz A-line rate with a motion tracking mechanism. A montage scanning protocol was used to capture a 100-degree field of view (FOV) using a 4×4 grid of sixteen total individual 6×6 mm2 scans. Typical OCTA images with a FOV of 3×3, 6×6 and 12×12 mm2 were obtained for comparison. DR patients were scanned at baseline and follow-up. They were treated at the clinician's discretion. Vessel density and non-perfusion area maps were calculated based on the UW-OCTA images.
Results: Three proliferative DR patients were included in the study. UW-OCTA images provided more detailed visualization of vascular networks compared to 50-degree fluorescein angiography (FA) and showed higher burden of pathology in the retinal periphery that was not captured by typical OCTA. Neovascularization complexes were clearly detected in the two patients with active PDR. Vessel density and non-perfusion maps were used to measure progressive capillary non-perfusion and regression of neovascularization between visits.
Conclusions: UW-OCTA provides approximately 100-degree OCTA images of the fundus comparable to that of wide-angle fundus photography, and may be more applicable in conditions such as DR which affect the peripheral retina in contrast to standard OCTA.
Keywords: Diabetic retinopathy (DR); field of view (FOV); non-perfusion; optical coherence tomography angiography (OCTA); swept source OCTA; vessel density.
Conflict of interest statement
Conflicts of Interest: Dr. Wang disclosed intellectual property owned by the Oregon Health and Science University and the University of Washington related to OCT angiography, and licensed to commercial entities, which are related to the technology and analysis methods described in parts of this manuscript. Dr. Wang received an innovative research award from Research to Prevent Blindness. He is a consultant to Carl Zeiss Meditec, and Insight Photonic Solutions.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
