Genomic and functional evidence reveals molecular insights into the origin of echolocation in whales
- PMID: 30306134
- PMCID: PMC6170035
- DOI: 10.1126/sciadv.aat8821
Genomic and functional evidence reveals molecular insights into the origin of echolocation in whales
Abstract
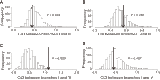
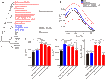
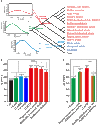
Echolocation allows toothed whales to adapt to underwater habitats where vision is ineffective. Because echolocation requires the ability to detect exceptional high-frequency sounds, fossils related to the auditory system can help to pinpoint the origin of echolocation in whales. However, because of conflicting interpretations of archaeocete fossils, when and how whales evolved the high-frequency hearing correlated with echolocation remain unclear. We address these questions at the molecular level by systematically investigating the convergent evolution of 7206 orthologs across 16 mammals and find that convergent genes between the last common ancestor of all whales (LCAW) and echolocating bats are not significantly enriched in functional categories related to hearing, and that convergence in hearing-related proteins between them is not stronger than that between nonecholocating mammalian lineages and echolocating bats. However, these results contrast with those of parallel analyses between the LCA of toothed whales (LCATW) and echolocating bats. Furthermore, we reconstruct the ancestral genes for the hearing protein prestin for the LCAW and LCATW; we show that the LCAW prestin exhibits the same function as that of nonecholocating mammals, but the LCATW prestin shows functional convergence with that of extant echolocating mammals. Mutagenesis shows that functional convergence of prestin is driven by convergent changes in the prestins S392A and L497M in the LCATW and echolocating bats. Our results provide genomic and functional evidence supporting the origin of high-frequency hearing in the LCAW, not the LCATW, and reveal molecular insights into the origin and evolutionary trajectories of echolocation in whales.
Figures


Similar articles
-
A functional enrichment test for molecular convergent evolution finds a clear protein-coding signal in echolocating bats and whales.Proc Natl Acad Sci U S A. 2019 Oct 15;116(42):21094-21103. doi: 10.1073/pnas.1818532116. Epub 2019 Sep 30. Proc Natl Acad Sci U S A. 2019. PMID: 31570615 Free PMC article.
-
Evolutionary Basis of High-Frequency Hearing in the Cochleae of Echolocators Revealed by Comparative Genomics.Genome Biol Evol. 2020 Jan 1;12(1):3740-3753. doi: 10.1093/gbe/evz250. Genome Biol Evol. 2020. PMID: 31730196 Free PMC article.
-
The hearing gene Prestin reunites echolocating bats.Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):13959-64. doi: 10.1073/pnas.0802097105. Epub 2008 Sep 5. Proc Natl Acad Sci U S A. 2008. PMID: 18776049 Free PMC article.
-
Functional convergence in bat and toothed whale biosonars.Physiology (Bethesda). 2013 Sep;28(5):276-83. doi: 10.1152/physiol.00008.2013. Physiology (Bethesda). 2013. PMID: 23997187 Review.
-
The evolution of echolocation in bats.Trends Ecol Evol. 2006 Mar;21(3):149-56. doi: 10.1016/j.tree.2006.01.001. Epub 2006 Feb 8. Trends Ecol Evol. 2006. PMID: 16701491 Review.
Cited by
-
Recurrent sequence evolution after independent gene duplication.BMC Evol Biol. 2020 Aug 8;20(1):98. doi: 10.1186/s12862-020-01660-1. BMC Evol Biol. 2020. PMID: 32770961 Free PMC article.
-
The conformational cycle of prestin underlies outer-hair cell electromotility.Nature. 2021 Dec;600(7889):553-558. doi: 10.1038/s41586-021-04152-4. Epub 2021 Oct 25. Nature. 2021. PMID: 34695838
-
Genomic Insights into the Adaptive Convergent Evolution.Curr Genomics. 2019 Feb;20(2):81-89. doi: 10.2174/1389202920666190313162702. Curr Genomics. 2019. PMID: 31555059 Free PMC article. Review.
-
Evolutionary sparse learning reveals the shared genetic basis of convergent traits.Nat Commun. 2025 Apr 4;16(1):3217. doi: 10.1038/s41467-025-58428-8. Nat Commun. 2025. PMID: 40185716 Free PMC article.
-
Molecular evolution of toothed whale genes reveals adaptations to echolocating in different environments.BMC Genomics. 2024 Nov 6;25(1):1049. doi: 10.1186/s12864-024-10910-1. BMC Genomics. 2024. PMID: 39506652 Free PMC article.
References
-
- McGowen M. R., Spaulding M., Gatesy J., Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Mol. Phylogenet. Evol. 53, 891–906 (2009). - PubMed
-
- Messenger S. L., McGuire J. A., Morphology, molecules, and the phylogenetics of cetaceans. Syst. Biol. 47, 90–124 (1998). - PubMed
-
- Nikaido M., Piskurek O., Okada N., Toothed whale monophyly reassessed by SINE insertion analysis: The absence of lineage sorting effects suggests a small population of a common ancestral species. Mol. Phylogenet. Evol. 43, 216–224 (2007). - PubMed
-
- Jones G., Echolocation. Curr. Biol. 15, R484–R488 (2005). - PubMed
-
- Churchill M., Martinez-Caceres M., de Muizon C., Mnieckowski J., Geisler J. H., The origin of high-frequency hearing in whales. Curr. Biol. 26, 2144–2149 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

