Dietary modifications for infantile colic
- PMID: 30306546
- PMCID: PMC6394439
- DOI: 10.1002/14651858.CD011029.pub2
Dietary modifications for infantile colic
Abstract
Background: Infantile colic is typically defined as full-force crying for at least three hours per day, on at least three days per week, for at least three weeks. This condition appears to be more frequent in the first six weeks of life (prevalence range of 17% to 25%), depending on the specific location reported and definitions used, and it usually resolves by three months of age. The aetiopathogenesis of infantile colic is unclear but most likely multifactorial. A number of psychological, behavioural and biological components (food hypersensitivity, allergy or both; gut microflora and dysmotility) are thought to contribute to its manifestation. The role of diet as a component in infantile colic remains controversial.
Objectives: To assess the effects of dietary modifications for reducing colic in infants less than four months of age.
Search methods: In July 2018 we searched CENTRAL, MEDLINE, Embase , 17 other databases and 2 trials registers. We also searched Google, checked and handsearched references and contacted study authors.
Selection criteria: Randomised controlled trials (RCTs) and quasi-RCTs evaluating the effects of dietary modifications, alone or in combination, for colicky infants younger than four months of age versus another intervention or placebo. We used specific definitions for colic, age of onset and the methods for performing the intervention. We defined 'modified diet' as any diet altered to include or exclude certain components.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Our primary outcome was duration of crying, and secondary outcomes were response to intervention, frequency of crying episodes, parental/family quality of life, infant sleep duration, parental satisfaction and adverse effects.
Main results: We included 15 RCTs involving 1121 infants (balanced numbers of boys and girls) aged 2 to 16 weeks. All studies were small and at high risk of bias across multiple design factors (e.g. selection, attrition). The studies covered a wide range of dietary interventions, and there was limited scope for meta-analysis. Using the GRADE approach, we assessed the quality of the evidence as very low.Low-allergen maternal diet versus a diet containing known potential allergens: one study (90 infants) found that 35/47 (74%) of infants responded to a low-allergen maternal diet, compared with 16/43 (37%) of infants on a diet containing known potential allergens.Low-allergen diet or soy milk formula versus dicyclomine hydrochloride: one study (120 infants) found that 10/15 (66.6%) breastfed babies responded to dicyclomine hydrochloride, compared with 24/45 (53.3%) formula-fed babies. There was little difference in response between breastfed babies whose mother changed their diet (10/16; 62.5%) and babies who received soy milk formula (29/44; 65.9%).Hydrolysed formula versus standard formula: two studies (64 infants) found no difference in duration of crying, reported as a dichotomous outcome: risk ratio 2.03, 95% confidence interval (CI) 0.81 to 5.10; very low-quality evidence. The author of one study confirmed there were no adverse effects. One study (43 infants) reported a greater reduction in crying time postintervention with hydrolysed formula (104 min/d, 95% CI 55 to 155) than with standard formula (3 min/d, 95% CI -63 to 67).Hydrolysed formula versus another hydrolysed formula: one study (22 infants) found that two types of hydrolysed formula were equally effective in resolving symptoms for babies who commenced with standard formula (Alimentum reduced crying to 2.21 h/d (standard deviation (SD) 0.40) and Nutramigen to 2.93 h/d (SD 0.70)).Hydrolysed formula or dairy- and soy-free maternal diet versus addition of parental education or counselling: one study (21 infants) found that crying time decreased to 2.03 h/d (SD 1.03) in the hydrolysed or dairy- and soy-free group compared with 1.08 h/d (SD 0.7) in the parent education or counselling group, nine days into the intervention.Partially hydrolysed, lower lactose, whey-based formulae containing oligosaccharide versus standard formula with simethicone: one study (267 infants) found that both groups experienced a decrease in colic episodes (secondary outcome) after seven days (partially hydrolysed formula: from 5.99 episodes (SD 1.84) to 2.47 episodes (SD 1.94); standard formula: from 5.41 episodes (SD 1.88) to 3.72 episodes (SD 1.98)). After two weeks the difference between the two groups was significant (partially hydrolysed: 1.76 episodes (SD 1.60); standard formula: 3.32 episodes (SD 2.06)). The study author confirmed there were no adverse effects.Lactase enzyme supplementation versus placebo: three studies (138 infants) assessed this comparison, but none reported data amenable to analysis for any outcome. There were no adverse effects in any of the studies.Extract of Foeniculum vulgare, Matricariae recutita, and Melissa officinalis versus placebo: one study (93 infants) found that average daily crying time was lower for infants given the extract (76.9 min/d (SD 23.5), than infants given placebo (169.9 min/d (SD 23.1), at the end of the one-week study. There were no adverse effects.Soy protein-based formula versus standard cows' milk protein-based formula: one study (19 infants) reported a mean crying time of 12.7 h/week (SD 16.4) in the soy formula group versus 17.3 h/week (SD 6.9) in the standard cows' milk group, and that 5/10 (50%) responded in the soy formula group versus 0/9 (0%) in the standard cows' milk group.Soy protein formula with polysaccharide versus standard soy protein formula: one study (27 infants) assessed this comparison but did not provide disaggregated data for the number of responders in each group after treatment.No study reported on our secondary outcomes of parental or family quality of life, infant sleep duration per 24 h, or parental satisfaction.
Authors' conclusions: Currently, evidence of the effectiveness of dietary modifications for the treatment of infantile colic is sparse and at significant risk of bias. The few available studies had small sample sizes, and most had serious limitations. There were insufficient studies, thus limiting the use of meta-analysis. Benefits reported for hydrolysed formulas were inconsistent.Based on available evidence, we are unable to recommend any intervention. Future studies of single interventions, using clinically significant outcome measures, and appropriate design and power are needed.
Conflict of interest statement
Morris Gordon (MG) received travel grants from a number of companies during the three years leading up to publication, including from Allergan, Ferring Pharmaceuticals, Biogaia, Synergy and Tillots, to attend conferences (Advances in Inflammatory Bowel Disease, Digestive Disease Week, World Congress of Gastroentrology) and present the results of other Cochrane Reviews. These companies have had no input or involvement in any aspect of the review process during this, or previous systematic reviews carried out by MG. MG currently holds a National Institute for Health Research (NIHR) Cochrane Programme Grant, although this did not support this work. MG is a paediatrician with an interest in gastroenterology. This involves seeing patients referred with infantile colic and managing their condition in line with current, accepted, evidence‐based practice. MG has no other interests to declare.
Elena Biagioli – none known.
Miriam Sorrenti – none known.
Carla Lingua – none known.
Lorenzo Moja – none known.
Shel Banks (SB) is self‐employed as an Internationally Board Certified Lactation Consultant in private practice, delivering expertise in infant feeding evidence base, writing briefing papers and newsletters, etc., and delivering workshops across the northwest of England – largely for the Local Infant Feeding Information Board (LIFIB), but also the Sudden Unexpected Death of a Child (SUDC) prevention team, Lanacshire. Until March 2016, the SUDC consultant post was funded by the local authority (Lancashire County Council) via breastfeeding voluntary sector charity (Breastfeeding Network) and SB was paid by the hour. SB is also self‐employed as a trainer and webinar presenter ad hoc and delivers Babyem's Open College Network Level Three and Four Maternity Nurse Training courses. Since 2009, SB has worked part‐time as a Baby Friendly Co‐ordinator, and she was paid for 12 months by Blackpool Teaching Hospitals NHS Foundation Trust as a Research Assistant for her work on this review. SB is Chair of LIFIB, Chair of the Communications Team, Chair of the Breastfeeding Festival, Committee Member of the Lactation Consultants of Great Britain, and a Trustee of the UK Association of Milk Banking, all of which are voluntary positions, although SB's travel expenses to meetings are paid. SB received travel expenses from Manchester City Council for speaking at their event 'Make Manchester a Breastfeeding Friendly City' in June 2018. SB has sat as a lay member on the Guideline Development Committee of three NICE Guidelines (Milk Banking, Postnatal Care Quality Standards and Faltering Growth), and was paid an honorarium by NICE (National Institute for Health and Care Excellence) for her services. SB declares that neither she personally, nor any of the entities she represents, take funding of any kind from any commercial interests in infant feeding or early years, and that she works completely within the professional code of ethics as an Internationally Board Certified Lactation Consultant. SB has no other interests to declare.
Simone Ceratto (SC) received travel grants from Nóos S.r.l and Nestlé, to attend scientific meetings. SC declares that he attended 'Campus Angelini 2017', which was sponsored by Angelini.
Francesco Savino (FS) received payment from: Danone Nutricia Middle‐East DMCC, Dubai, United Arab Emirates, and Innova Pharma SpA Recordati SpA, Milan, Italy, for talks at symposiums; Società Italiana di Pediatria, Milan, Italy, for speaking at the XX Congresso Nazionale SIAIP, sponsored by Biomedia srl, Milan; HiPP GmbH & Co, Vertrieb KG, Pfaffenhofen, Germany, for consultancy work; and from Aretrè srl, Milan, Italy, for development of educational materials, outside the submitted work. FS also received a travel grant from Noos Roma, Italy, to attend ESPGHAN's 51st Annual Meeting, Geneva, and receives royalties from Springer Verlag, Milan, Italy, for the book
Disclaimer: the views contained herein are those of the authors and not necessarily those of the Department of Health, NHS NICE or NIHR.
Figures
Update of
References
References to studies included in this review
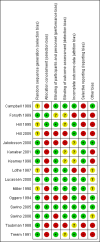
Campbell 1989 {published data only}
Forsyth 1989 {published data only}
-
- Forsyth BW. Colic and the effect of changing formulas: a double‐blind, multiple‐crossover study. Journal of Pediatrics 1989;115(4):521‐6. [PUBMED: 2677292] - PubMed
Hill 1995 {published data only}
-
- Hill DJ, Hudson IL, Sheffiled LJ, Shelton MJ, Menahem S, Hosking CS. A low allergen diet is a significant intervention in infantile colic: results of a community‐based study. Journal of Allergy and Clinical Immunology 1995;96(6 Pt 1):886‐92. [PUBMED: 8543745] - PubMed
Hill 2005 {published data only}
-
- Hill DJ, Hosking CS, Roy N, Carlin JB, Francis DEM, Brown J, et al. Colic in breast fed infants is due to hypersensitivity to dietary proteins excreted in breast milk. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S338. [DOI: 10.1016/j.jaci.2004.01.724] - DOI
Jakobsson 2000 {published data only}
Kanabar 2001 {published data only}
-
- Kanabar D, Randhawa M, Clayton P. Improvement of symptoms in infant colic following reduction of lactose load with lactase. Journal of Human Nutrition and Dietetics 2001;14(5):359‐63. [PUBMED: 11906576] - PubMed
Kearney 1998 {published data only}
-
- Kearney PJ, Malone AJ, Hayes T, Cole M, Hyland M. A trial of lactase in the management of infant colic. Journal of Human Nutrition and Dietetics 1998;11(4):281‐5. [DOI: 10.1046/j.1365-277X.1998.00108.x] - DOI
Lothe 1987 {published data only}
-
- Lothe L, Lindberg T, Jakobsson I. Cow's milk formula as a cause of infantile colic: a double‐blind study. Pediatrics 1982;70(1):7‐10. [PUBMED: 7088636] - PubMed
Lucassen 2000 {published data only}
-
- Lucassen P. Cochrane dietary modifications for colic review [personal communication]. Email to: M Gordon 8 November 2016.
-
- Lucassen PL, Assendelft WJ, Gubbels JW, Eijk JT, Douwes AC. Infantile colic: crying time reduction with a whey hydrolysate: a double‐blind, randomized, placebo‐controlled trial. Pediatrics 2000;106(6):1349‐54. [PUBMED: 11099588] - PubMed
Miller 1990 {published data only}
-
- Miller J. Details for our Cochrane review [personal communication]. Email to: S Banks 8 November 2016.
-
- Miller JJ, McVeagh P, Fleet GH, Petocz, P, Brand JC. Effect of yeast lactase enzyme on "colic" in infants fed human milk. Journal of Pediatrics 1990;117(2 Pt 1):261‐3. [PUBMED: 2116510] - PubMed
Oggero 1994 {published data only}
-
- Oggero R, Garbo G, Savino F, Mostert M. Dietary modifications versus dicyclomine hydrochloride in the treatment of severe infantile colics. Acta Paediatrica 1994;83(2):222‐5. [PUBMED: 8193507] - PubMed
Savino 2005 {published data only}
-
- Savino F, Cresi F, Castagno E, Silvestro L, Oggero R. A randomized double‐blind placebo‐controlled trial of a standardized extract of matricariae recutita, foeniculum vulgare and melissa officinalis (ColiMil) in the treatment of breastfed colicky infants. Phytotherapy Research 2005;19(4):335‐40. [DOI: 10.1002/ptr.1668; PUBMED: 16041731] - DOI - PubMed
Savino 2006 {published data only}
-
- Savino F. Confirmation of details from your review [personal communication]. Email to: M Gordon 15 December 2016.
-
- Savino F, Palumeri E, Castagno E, Cresi F, Dalmasso P, Cavallo F, et al. Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. European Journal of Clinical Nutrition 2006;60(11):1304‐10. [DOI: 10.1038/sj.ejcn.1602457; PUBMED: 16736065] - DOI - PubMed
Taubman 1988 {published data only}
-
- Taubman B. Parental counseling compared with elimination of cow's milk or soy milk protein for the treatment of infant colic syndrome: a randomized trial. Pediatrics 1988;81(6):756‐61. [PUBMED: 3285312] - PubMed
Treem 1991 {published data only}
-
- Treem WR, Hyams JS, Blankschen E, Etienne N, Paule CL, Borschel MW. Evaluation of the effect of a fiber‐enriched formula on infant colic. Journal of Pediatrics 1991;119(5):695‐701. [PUBMED: 1658281] - PubMed
References to studies excluded from this review
Arikan 2008 {published data only}
-
- Arikan D, Alp H, Gözüm S, Orbak Z, Cifçi EK. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. Journal of Clinical Nursing 2008;17(13):1754‐61. [PUBMED: 18592627] - PubMed
Barr 1991 {published data only}
-
- Barr RG, Wooldridge J, Hanley J. Effects of formula change on intestinal hydrogen production and crying and fussing behavior. Journal of Developmental and Behavioral Pediatrics 1991;12(4):248‐53. [PUBMED: 1939680] - PubMed
-
- Barr RG, Wooldridge JA, Hanley J, Jajko L, Boisjoly C. Formula change reduces crying behavior: evidence for role of intraintestinal gas. Pediatric Research 1984;18:101A. [DOI: 10.1203/00006450-198404001-00047] - DOI
Bellaiche 2018 {published data only}
-
- Bellaiche M, Oozeer R, Gerardi‐Temporel G, Faure C, Vandenplas Y. Multiple functional gastrointestinal disorders are frequent in formula‐fed infants and decrease their quality of life. Acta Paediatrica 2018;107(7):1276‐82. [DOI: 10.1111/apa.14348; MEDLINE: ; PMC6055647; PUBMED: 29604128] - DOI - PMC - PubMed
Berseth 2009 {published data only}
Buchanan 1998 {published data only}
-
- Buchanan P. Effectiveness of treatments for infantile colic. Trial of hypoallergenic milk is not supported by strong enough evidence. BMJ 1998;317(7170):1451‐2. [PUBMED: 10232901] - PubMed
Campeotto 2011 {published data only}
-
- Campeotto F, Suau A, Kapel N, Magne F, Viallon V, Ferraris L, et al. A fermented formula in pre‐term infants: clinical tolerance, gut microbiota, down‐regulation of faecal calprotectin and up‐regulation of faecal secretory IgA. British Journal of Nutrition 2011;105(12):1843‐51. [DOI: 10.1017/S0007114510005702; PUBMED: 21426607] - DOI - PubMed
Dupont 2010 {published data only}
-
- Dupont C, Rivero M, Grillon C, Belaroussi N, Kalindjian A, Marin V. Alpha‐lactalbumin‐enriched and probiotic‐supplemented infant formula in infants with colic: growth and gastrointestinal tolerance. European Journal of Clinical Nutrition 2010;64(7):765‐7. [DOI: 10.1038/ejcn.2010.81; PUBMED: 20517331] - DOI - PubMed
Evans 1981 {published data only}
-
- Evans RW, Fergusson DM, Allardyce RA, Taylor B. Maternal diet and infantile colic in breast‐fed infants. Lancet 1981;1(8234):1340‐2. [PUBMED: 6113313] - PubMed
Gerrard 1984 {published data only}
Giovannini 2014 {published data only}
-
- Giovannini M, Verduci E, Gregori D, Ballali S, Soldi S, Ghisleni D, et al. Prebiotic effect of an infant formula supplemented with galacto‐oligosaccharides: randomized multicenter trial. Journal of the American College of Nutrition 2014;33(5):385‐93. [DOI: 10.1080/07315724.2013.878232; PUBMED: 25302927] - DOI - PubMed
Iacono 1991 {published data only}
-
- Iacono G, Carroccio A, Montalto G, Cavataio F, Bragion E, Lorello D, et al. Severe infantile colic and food intolerance: a long‐term prospective study. Journal of Pediatric Gastroenterology and Nutrition 1991;12(3):332‐5. [PUBMED: 2072224] - PubMed
Iacovou 2018 {published data only}
-
- Iacovou M, Mulcahy EC, Truby H, Barrett JS, Gibson PR, Muir JG. Reducing the maternal dietary intake of indigestible and slowly absorbed short‐chain carbohydrates is associated with improved infantile colic: a proof‐of‐concept study. Journal of Human Nutrition and Dietetics 2018;31(2):256‐65. [DOI: 10.1111/jhn.12488; PUBMED: 28631347] - DOI - PubMed
Imanieh 2004 {published data only}
-
- Imanieh MH, Moravej H, Kashef S, Handjani F, Eghtedari F, Kamali Sarvestani E. Cows' milk allergy in infantile colic. Armaghane Danesh 2004;9(3):39‐47. [armaghanj.yums.ac.ir/article‐1‐816‐en.html]
Infante 2011 {published data only}
Jakobsson 1983 {published data only}
-
- Jakobsson I, Lindberg T. Cow's milk proteins cause infantile colic in breast‐fed infants: a double‐blind crossover study. Pediatrics 1983;71(2):268‐71. [PUBMED: 6823433] - PubMed
Koonce 2011 {published data only}
Laws 1991 {published data only}
Liebman 1981 {published data only}
-
- Liebman WM. Infantile colic: association with lactose and milk intolerance. JAMA 1981;245(7):732‐3. [PUBMED: 7193254] - PubMed
Nocerino 2012 {published data only}
-
- Nocerino R, Marco G, Cosenza L, Pezzella V, Passariello A, Terrin G, et al. Efficacy of a new partially hydrolyzed whey formula on infant colic: a randomized controlled trial. Digestive and Liver Disease 2012;44(Suppl 4):S272. [DOI: 10.1016/S1590-8658(12)60696-3; P032] - DOI
Pärtty 2015 {published data only}
Rozé 2012 {published data only}
-
- Rozé JC, Barbarot S, Butel MJ, Kapel N, Waligora‐Dupriet AJ, Montgolfier I, et al. An α‐lactalbumin‐enriched and symbiotic‐supplemented v a standard infant formula: a multicentre, double‐blind, randomised trial. British Journal of Nutrition 2012;107(11):1616–22. [DOI: 10.1017/S000711451100479X; NCT00920166; PUBMED: 22079177] - DOI - PubMed
Sargsyan 2006 {published data only}
-
- Sargsyan K, Burmucie K, Dunitz‐Scheer M, Hauer AC. Effects of nutritional and psychosupportive interventions on the course of infantile colic ‐ first results of a prospective clinical study. European Journal of Pediatrics 2006;165(Suppl 1):360. [DOI: 10.1007/s00431-006-0349-z] - DOI
Savilahti 1989 {published data only}
Savino 2003 {published data only}
-
- Savino F, Cresi F, Maccario S, Cavallo F, Dalmasso P, Fanaro S, et al. "Minor" feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo‐ and galacto oligosaccharides. Acta Pediatrica 2003;91(441 Suppl):86‐90. [PUBMED: 14599049] - PubMed
Sherman 2015 {published data only}
-
- Sherman AL, Anderson J, Rudolph CD, Walker LS. Lactose‐free milk or soy‐based formulas do not improve caregivers' distress or perceptions of difficult infant behavior. Journal of Pediatric Gastroenterology and Nutrition 2015;61(1):119‐24. [DOI: 10.1097/MPG.0000000000000743; 25643020; NCT01934257] - DOI - PubMed
Vandenplas 2017 {published data only}
-
- Vandenplas Y, Ludwig T, Bouritius H, Alliet P, Forde D, Peeters S, et al. Randomised controlled trial demonstrates that fermented infant formula with short‐chain galacto‐oligosaccharides and long‐chain fructo‐oligosaccharides reduces the incidence of infantile colic. Acta Paediatrica 2017;106(7):1150‐8. [DOI: 10.1111/apa.13844; PMC5485044; PUBMED: 28328065] - DOI - PMC - PubMed
Vivatvakin 2010 {published data only}
-
- Vivatvakin B, Mahayosnond A, Theamboonlers A, Steenhout PG, Conus NJ. Effect of a whey‐predominant starter formula containing LCPUFAs and oligosaccharides (FOS/GOS) on gastrointestinal comfort in infants. Asia Pacific Journal of Clinical Nutrition 2010;19(4):473‐80. [PUBMED: 21147707] - PubMed
Xinias 2017 {published data only}
References to ongoing studies
NCT01721850 {unpublished data only}
-
- NCT01721850. Evaluation of safety and efficacy of new infant formula in infantile colic (Coco) [Evaluation of the safety and efficacy of new infant formula and its effects on the gastrointestinal tolerance (crying time) in infantile colic: a double‐blind, randomized, controlled intervention study]. clinicaltrials.gov/ct2/show/NCT01721850 (first received 6 November 2012).
NCT02813772 {unpublished data only}
-
- NCT02813772. Efficacy of a partially hydrolyzed formula, containing Lactobacillus reuteri, for infant colic [Efficacy of a partially hydrolyzed formula, containing Lactobacillus reuteri, for infant colic: a double blind, randomized‐controlled trial]. clinicaltrials.gov/ct2/show/NCT02813772 (first received 27 June 2016).
NCT03329222 {unpublished data only}
-
- NCT03329222. An infant formula trial on dietary management of infantile colic [A randomised, double blind, controlled, multi‐centre study to assess the efficacy of an infant formula in the dietary management of infantile colic]. clinicaltrials.gov/ct2/show/NCT03329222?term=NCT03329222&rank=1 (first received 1 November 2017).
Additional references
Agostoni 2006
-
- ESPGHAN Committee on Nutrition, Agostoni C, Axelsson I, Goulet O, Koletzko B, Michaelsen KF, et al. Soy protein infant formulae and follow‐on formulae: a commentary by the ESPGHAN Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2006;42(4):352‐61. [DOI: 10.1097/01.mpg.0000189358.38427.cd; PUBMED: 16641572] - DOI - PubMed
Axelsson 1986
-
- Axelsson I, Jakobsson I, Lindberg T, Benediktsson B. Bovine beta‐lactoglobulin in the human milk. A longitudinal study during the whole lactation period. Acta Paediatrica Scandinavica 1986;75(5):702‐7. [PUBMED: 3564937] - PubMed
Benninga 2016
Camilleri 2017
Clifford 2002
-
- Clifford TJ, Campbell MK, Speechley KN, Gorodzinsky F. Infant colic: empirical evidence of the absence of an association with source of early infant nutrition. Archives of Pediatrics & Adolescent Medicine 2002;156(11):1123‐8. [PUBMED: 12413341] - PubMed
Clyne 1991
-
- Clyne PS, Kulczycki A Jr. Human breast milk contains bovine IgG. Relationship to infant colic?. Pediatrics 1991;87(4):439‐44. [PUBMED: 2011419] - PubMed
Cohen‐Silver 2009
de Weerth 2013
Deeks 2017
-
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Donaldson 2004
-
- Donaldson L. Advice issued on soya‐based infant formulas. CMO Update 2004; Vol. 37:2. [www.second‐opinions.co.uk/soy‐online‐service/04070176.pdf]
Drossman 2016
Dubois 2016
Elbourne 2002
Estep 2000
-
- Estep DC, Kulczycki A Jr. Treatment of infant colic with amino acid‐based infant formula: a preliminary study. Acta Paediatrica 2000;89(1):22‐7. [PUBMED: 10677052] - PubMed
Farré 2013
Feinle‐Bisset 2013
Freedman 2009
García Marqués 2017
Garrison 2000
-
- Garrison MM, Christakis DA. A systematic review of treatments for infant colic. Pediatrics 2000;106(1 Pt 2):184‐90. [PUBMED: 10888690] - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 30 July 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gupta 2007
-
- Gupta SK. Update on infantile colic and management options. Current Opinion in Investigational Drugs 2007;8(11):921‐6. [PUBMED: 17979025] - PubMed
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI: 10.1136/bmj.39489.470347.AD; PMC2335261; PUBMED: 18436948] - DOI - PMC - PubMed
Hall 2012
Heine 2006
Heine 2008
Higgins 2011a
-
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hill 2000
-
- Hill DJ, Hosking CS. Infantile colic and food hypersensitivity. Journal of Pediatric Gastroenterology and Nutrition 2000;30 Suppl:S67‐76. [PUBMED: 10634302] - PubMed
Hyams 1989
-
- Hyams JS, Geertsma MA, Etienne NL, Treem WR. Colonic hydrogen production in infants with colic. Journal of Pediatrics 1989;115(4):592‐4. [PUBMED: 2795353] - PubMed
Iacono 2005
Iacovou 2012
Keefe 1998
-
- Keefe MR, Froese‐Fretz A, Kotzer AM. Newborn predictors of infant irritability. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1998;27(5):513‐20. [PUBMED: 9773363] - PubMed
Landgren 2010
Lindberg 1999
-
- Lindberg T. Infantile colic and small intestinal function: a nutritional problem?. Acta Paediatrica 1999;88(430):58‐60. [PUBMED: 10569224] - PubMed
Lothe 1989
-
- Lothe L, Lindberg T. Cow's milk whey protein elicits symptoms of infantile colic in colicky formula‐fed infants: a double‐blind crossover study. Pediatrics 1989;83(2):262‐6. [PUBMED: 2913556] - PubMed
Lothe 1990
-
- Lothe L, Ivarsson SA, Ekman R, Lindberg T. Motilin and infantile colic. A prospective study. Acta Paediatrica Scandinavica 1990;79(4):410‐6. [PUBMED: 2349877] - PubMed
Lucas 1998
-
- Lucas A, James‐Roberts I. Crying, fussing and colic behaviour in breast‐ and bottle‐fed infants. Early Human Development 1998;53(1):9‐18. [PUBMED: 10193923] - PubMed
Lucassen 1998
Lucassen 2001
Moher 2009
Moore 1988
-
- Moore DJ, Robb TA, Davidson GP. Breath hydrogen response to milk containing lactose in colicky and noncolicky infants. Journal of Pediatrics 1988;113(6):979‐84. [PUBMED: 3193321] - PubMed
Osborn 2013
Oxman 1992
Pauli‐Pott 2000
-
- Pauli‐Potta U, Becker K, Mertesacker T, Beckmann D. Infants with "colic" ‐ mothers' perspectives on the crying problem. Journal of Psychosomatic Research 2000;48(2):125‐32. [PUBMED: 10719128] - PubMed
Perry 2011
Pitkin 1999
-
- Pitkin RM, Branagan MA, Burmeister LF. Accuracy of data in abstracts of published research articles. JAMA 1999;281(12):1110‐1. [PUBMED: 10188662] - PubMed
Praveen 2014
-
- Praveen V, Praveen S, Deshpande G, Patole SK. Oral probiotics for infantile colic. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD010986] - DOI
Reijneveld 2001
-
- Reijneveld SA, Brugman E, Hirasing RA. Excessive infant crying: the impact of varying definitions. Pediatrics 2001;108(4):893‐7. [PUBMED: 11581441] - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaberation. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaberation, 2014.
Savino 2001
-
- Savino F, Cresi F, Silvestro L, Oggero R. Use of an amino‐acid formula in the treatment of colicky breastfed infants. Acta Paediatrica 2001;90(3):359‐60. [PUBMED: 11332183] - PubMed
Savino 2007
Savino 2010
Savino 2017
-
- Savino F, Quartieri A, Marco A, Garro M, Amaretti A, Raimondi S, et al. Comparison of formula‐fed infants with and without colic revealed significant differences in total bacteria, enterobacteriaceae and faecal ammonia. Acta Paediatrica 2017;106(4):573‐8. [DOI: 10.1111/apa.13642; PUBMED: 27763733] - DOI - PubMed
Schach 2002
Shannon 1921
-
- Shannon WR. Colic in breast fed infants as a result of sensitization to foods in the mother's diet. Archives of Pediatrics 1921;38:756‐61.
Steutel 2014
Sung 2014
Thomas 2010
Vandenplas 2015
-
- Vandenplas Y, Abkari A, Bellaiche M, Benninga M, Chouraqui JP, Çokura F, et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. Journal of Pediatric Gastroenterology and Nutrition 2015;61(5):531‐7. [DOI: 10.1097/MPG.0000000000000949; PMC4631121; PUBMED: 26308317] - DOI - PMC - PubMed
Walter 2000
-
- Walter SD. Choice of effect measure for epidemiological data. Journal of Clinical Epidemiology 2000;53(9):931‐9. [PUBMED: 11004419] - PubMed
Wessel 1954
-
- Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics 1954;14(5):421‐35. [PUBMED: 13214956] - PubMed
Wolke 2017
Yusuf 1991
-
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266(1):93–8. [PUBMED: 2046134] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical