Cellular polarity modulates drug resistance in primary colorectal cancers via orientation of the multidrug resistance protein ABCB1
- PMID: 30306567
- PMCID: PMC6519031
- DOI: 10.1002/path.5179
Cellular polarity modulates drug resistance in primary colorectal cancers via orientation of the multidrug resistance protein ABCB1
Abstract
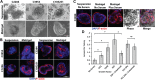
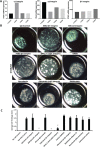
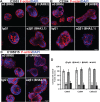
Colonic epithelial cells are highly polarised with a lumen-facing apical membrane, termed the brush border, and a basal membrane in contact with the underlying extracellular matrix (ECM). This polarity is often maintained in cancer tissue in the form of neoplastic glands and has prognostic value. We compared the cellular polarity of several ex vivo spheroid colonic cancer cultures with their parental tumours and found that those grown as non-attached colonies exhibited apical brush border proteins on their outer cellular membranes. Transfer of these cultures to an ECM, such as collagen, re-established the centralised apical polarity observed in vivo. The multidrug resistance protein ABCB1 also became aberrantly polarised to outer colony membranes in suspension cultures, unlike cultures grown in collagen, where it was polarised to central lumens. This polarity switch was dependent on the presence of serum or selected serum components, including epidermal growth factor (EGF), transforming growth factor-β1 (TGF-β1) and insulin-like growth factor-1 (IGF-1). The apical/basal orientation of primary cancer colon cultures cultured in collagen/serum was modulated by α2β1 integrin signalling. The polarisation of ABCB1 in colonies significantly altered drug uptake and sensitivity, as the outward polarisation of ABCB1 in suspension colonies effluxed substrates more effectively than ECM-grown colonies with ABCB1 polarised to central lumens. Thus, serum-free suspension colonies were more resistant to a variety of anti-cancer drugs than ECM-grown colonies. In conclusion, the local stroma, or absence thereof, can have profound effects on the sensitivity of colorectal cultures to drugs that are ABCB1 substrates. © 2018 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: ABCB1; colon; drug resistance; polarity; primary culture; spheroid.
© 2018 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures



References
-
- Furuta S, Bissell MJ. Pathways involved in formation of mammary organoid architecture have keys to understanding drug resistance and to discovery of druggable targets. Cold Spring Harb Symp Quant Biol 2016; 81: 207–217. - PubMed
-
- Manninen A. Epithelial polarity – generating and integrating signals from the ECM with integrins. Exp Cell Res 2015; 334: 337–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

