ATP promotes immunosuppressive capacities of mesenchymal stromal cells by enhancing the expression of indoleamine dioxygenase
- PMID: 30306723
- PMCID: PMC6247240
- DOI: 10.1002/iid3.236
ATP promotes immunosuppressive capacities of mesenchymal stromal cells by enhancing the expression of indoleamine dioxygenase
Abstract
Introduction: MSCs are often found within tumors, promote cancer progression and enhance metastasis. MSCs can act as immuosuppressive cells, partially due to the expression of the enzyme indoleamine dioxygenase (IDO) which converts tryptophan to kynurenine. Decreased concentration of tryptophan and increased kynurenine, both interfere with effective immune response. Damage associated molecular patterns (DAMPs) including ATP are found within the tumor microenvironment, attract MSCs, and influence their biology.
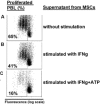
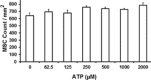
Methods: Bone marrow derived MSCs were exposed to ATP for 4 days, in the presence of 100 ng IFNγ/mL. Intracellular expression of IDO in MSCs was assessed by FACS. Conditioned media from thus stimulated MSCs was analyzed for kynurenine content and its suppressive effect on lymphocyte proliferation. Apyrase or P2 × 7-receptor antagonist (AZ 11645373) were applied in order to inhibit ATP induced effect on MSCs.
Results: We demonstrate, that ATP at concentrations between 0.062 and 0.5 mM increases dose dependently the expression of IDO in MSCs with subsequent increased kynurenine concentrations within the supernatant at about 60%. This effect could be abolished completely in the presence of ATP degrading enzyme (apyrase) or when MSCs were pretreated with a P2 × 7-receptor antagonist (AZ 11645373). Consistently, supernatants from MSCs stimulated with ATP, inhibited lymphocyte proliferation from 65% to 16%.
Conclusions: We characterized ATP as a DAMP family member responsible for necrosis-induced immunomodulation. Given the increased concentration of DAMPs within tumor tissue and the fact that DAMPs can act as chemotattractants to MSCs, our results have implications for therapeutic strategies targeting the tumor microenvironment.
Keywords: DAMPs; cancer; inflammation; mesenchymal stromal cells; wound healing.
© 2018 The Authors. Immunity, Inflammation and Disease Published by John Wiley & Sons Ltd.
Figures



Similar articles
-
Indoleamine 2,3-dioxygenase mediates inhibition of virus-specific CD8(+) T cell proliferation by human mesenchymal stromal cells.Cytotherapy. 2016 May;18(5):621-9. doi: 10.1016/j.jcyt.2016.01.009. Cytotherapy. 2016. PMID: 27008974
-
Immune cell proliferation is suppressed by the interferon-gamma-induced indoleamine 2,3-dioxygenase expression of fibroblasts populated in collagen gel (FPCG).J Cell Biochem. 2003 Sep 1;90(1):206-17. doi: 10.1002/jcb.10593. J Cell Biochem. 2003. PMID: 12938169
-
Interferon-γ enhances the immunosuppressive ability of canine bone marrow-derived mesenchymal stem cells by activating the TLR3-dependent IDO/kynurenine pathway.Mol Biol Rep. 2022 Sep;49(9):8337-8347. doi: 10.1007/s11033-022-07648-y. Epub 2022 Jun 12. Mol Biol Rep. 2022. PMID: 35690960
-
Indoleamine-2,3 dioxygenase: a fate-changer of the tumor microenvironment.Mol Biol Rep. 2023 Jul;50(7):6133-6145. doi: 10.1007/s11033-023-08469-3. Epub 2023 May 22. Mol Biol Rep. 2023. PMID: 37217614 Free PMC article. Review.
-
Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway.Int J Biochem Cell Biol. 2009 Mar;41(3):467-71. doi: 10.1016/j.biocel.2008.01.005. Epub 2008 Jan 11. Int J Biochem Cell Biol. 2009. PMID: 18282734 Review.
Cited by
-
Role of indoleamine 2,3-dioxygenase in ischemia-reperfusion injury of renal tubular epithelial cells.Mol Med Rep. 2021 Jun;23(6):472. doi: 10.3892/mmr.2021.12111. Epub 2021 Apr 26. Mol Med Rep. 2021. PMID: 33899121 Free PMC article.
-
Mesenchymal Stromal Cell Immunology for Efficient and Safe Treatment of Osteoarthritis.Front Cell Dev Biol. 2020 Sep 22;8:567813. doi: 10.3389/fcell.2020.567813. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33072752 Free PMC article. Review.
-
Roles of extracellular adenosine triphosphate on the functions of periodontal ligament cells.BDJ Open. 2023 Jul 8;9(1):28. doi: 10.1038/s41405-023-00147-7. BDJ Open. 2023. PMID: 37422449 Free PMC article. Review.
-
Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies.Clin Transl Med. 2020 Jan;10(1):199-223. doi: 10.1002/ctm2.24. Clin Transl Med. 2020. PMID: 32508035 Free PMC article. Review.
-
Mesenchymal Stem Cells and Purinergic Signaling in Autism Spectrum Disorder: Bridging the Gap between Cell-Based Strategies and Neuro-Immune Modulation.Biomedicines. 2024 Jun 13;12(6):1310. doi: 10.3390/biomedicines12061310. Biomedicines. 2024. PMID: 38927517 Free PMC article. Review.
References
-
- Zeh HJ, III , Lotze MT. Addicted to death: invasive cancer and the immune response to unscheduled cell death. J Immunother. 2005; 28:1–9. - PubMed
-
- Lotfi R, Eisenbacher J, Solgi G, et al. Human mesenchymal stem cells respond to native but not oxidized damage associated molecular pattern molecules from necrotic (tumor) material. Eur J Immunol. 2011; 41:2021–2028. - PubMed
-
- Lotfi R, Wiegmann DS, Asseck L, et al. Necrosis‐associated factors (DAMPs) including S100A4 used to pulse dendritic cells (DCs) induce regulatory T cells. J Cell Sci Ther. 3:134.
-
- Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004; 4:641–648. - PubMed
-
- Young HE, Steele TA, Bray RA, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001; 264:51–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

