The Relationship Between Blood Sample Volume and Diagnostic Sensitivity of Blood Culture for Typhoid and Paratyphoid Fever: A Systematic Review and Meta-Analysis
- PMID: 30307563
- PMCID: PMC6226661
- DOI: 10.1093/infdis/jiy471
The Relationship Between Blood Sample Volume and Diagnostic Sensitivity of Blood Culture for Typhoid and Paratyphoid Fever: A Systematic Review and Meta-Analysis
Abstract
Background: Blood culture is the standard diagnostic method for typhoid and paratyphoid (enteric) fever in surveillance studies and clinical trials, but sensitivity is widely acknowledged to be suboptimal. We conducted a systematic review and meta-analysis to examine sources of heterogeneity across studies and quantified the effect of blood volume.
Methods: We searched the literature to identify all studies that performed blood culture alongside bone marrow culture (a gold standard) to detect cases of enteric fever. We performed a meta-regression analysis to quantify the relationship between blood sample volume and diagnostic sensitivity. Furthermore, we evaluated the impact of patient age, antimicrobial use, and symptom duration on sensitivity.
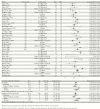
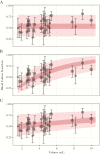
Results: We estimated blood culture diagnostic sensitivity was 0.59 (95% confidence interval [CI], 0.54-0.64) with significant between-study heterogeneity (I2, 76% [95% CI, 68%-82%]; P < .01). Sensitivity ranged from 0.51 (95% CI, 0.44-0.57) for a 2-mL blood specimen to 0.65 (95% CI, 0.58-0.70) for a 10-mL blood specimen, indicative of a relationship between specimen volume and sensitivity. Subgroup analysis showed significant heterogeneity by patient age and a weak trend towards higher sensitivity among more recent studies. Sensitivity was 34% lower (95% CI, 4%-54%) among patients with prior antimicrobial use and 31% lower after the first week of symptoms (95% CI, 19%-41%). There was no evidence of confounding by patient age, antimicrobial use, symptom duration, or study date on the relationship between specimen volume and sensitivity.
Conclusions: The relationship between the blood sample volume and culture sensitivity should be accounted for in incidence and next-generation diagnostic studies.
Figures


References
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1545–602. - PMC - PubMed
-
- Crump JA. Typhoid fever and the challenge of nonmalaria febrile illness in sub-Saharan Africa. Clin Infect Dis 2012; 54:1107–9. - PubMed
-
- World Health Organization. Background document: the diagnosis, treatment and prevention of typhoid fever. 2003. http://www.who.int/iris/handle/10665/68122. Accessed January 2018.
-
- Gilman RH, Terminel M, Levine MM, Hernandez-Mendoza P, Hornick RB. Relative efficacy of blood, urine, rectal swab, bone-marrow, and rose-spot cultures for recovery of Salmonella typhi in typhoid fever. Lancet 1975; 1:1211–3. - PubMed

