Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage
- PMID: 30307611
- PMCID: PMC6515924
- DOI: 10.1002/cncr.31720
Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage
Abstract
Background: The outcomes of patients with relapsed or refractory (R-R) acute lymphoblastic leukemia (ALL) are poor. Inotuzumab ozogamicin and blinatumomab have single-agent activity in R-R ALL. Their addition to low-intensity chemotherapy may further improve the outcomes of patients with ALL in their first relapse.
Methods: The chemotherapy was lower in intensity than conventional hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone and was called mini-hyperfractionated cyclophosphamide, vincristine, and dexamethasone (or mini-HCVD). Inotuzumab was given on day 3 of each of the first 4 cycles at 1.8 to 1.3 mg/m2 for cycle 1, and this was followed by 1.3 to 1.0 mg/m2 for subsequent cycles. From patient 39 onward, the inotuzumab dose was reduced and fractionated into weekly doses (0.6 and 0.3 mg/m2 during cycle 1 and 0.3 and 0.3 mg/m2 during subsequent cycles), and blinatumomab was administered for up to 4 cycles after inotuzumab therapy.
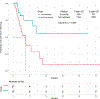
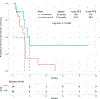
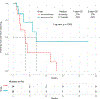
Results: Forty-eight patients with Philadelphia chromosome-negative ALL with a median age of 39 years were treated during their first relapse. Overall, 44 patients (92%) responded, with 35 of them (73%) achieving a complete response. The overall minimal residual disease negativity rate among the responders was 93%. Twenty-four patients (50%) underwent allogeneic stem cell transplantation (ASCT). Veno-occlusive disease of any grade occurred in 5 patients (10%). With a median follow-up of 31 months, the median progression-free survival (PFS) and the median overall survival (OS) were 11 and 25 months, respectively. The 2-year PFS and OS rates were 42% and 54%, respectively. Of the 24 patients (50%) who underwent ASCT, 14 patients were alive at the last follow-up (13 [54%] in remission). Of the remaining 20 responding patients who did not undergo subsequent ASCT, 6 (30%) remained in remission at the last follow-up. According to propensity score matching, the combination of mini-HCVD and inotuzumab with or without blinatumomab conferred better outcomes than intensive salvage chemotherapy or inotuzumab alone.
Conclusions: The combination of inotuzumab and low-intensity mini-HCVD chemotherapy with or without blinatumomab shows encouraging results in patients with ALL in first salvage.
Keywords: acute lymphoblastic leukemia (ALL); first relapse; inotuzumab ozogamicin; salvage.
© 2018 American Cancer Society.
Conflict of interest statement
Figures




References
-
- Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–50. - PubMed

