A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid
- PMID: 30307935
- PMCID: PMC6198998
- DOI: 10.1371/journal.pntd.0006779
A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid
Abstract
Background: The temporal and spatial change in trends of antimicrobial resistance (AMR) in typhoid have not been systematically studied, and such information will be critical for defining intervention, as well as planning sustainable prevention strategies.
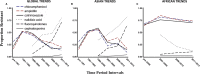
Methodology and findings: To identify the phenotypic trends in AMR, 13,833 individual S. Typhi isolates, reported from 1973 to 2018 in 62 publications, were analysed to determine the AMR preponderance over time. Separate analyses of molecular resistance determinants present in over 4,000 isolates reported in 61 publications were also conducted. Multi-drug resistant (MDR) typhoid is in decline in Asia in a setting of high fluoroquinolone resistance while it is on the increase in Africa. Mutations in QRDRs in gyrA (S83F, D87N) and parC (S80I) are the most common mechanisms responsible for fluoroquinolone resistance. Cephalosporin resistant S. Typhi, dubbed extensively drug-resistant (XDR) is a real threat and underscores the urgency in deploying the Vi-conjugate vaccines.
Conclusion: From these observations, it appears that AMR in S. Typhi will continue to emerge leading to treatment failure, changes in antimicrobial policy and further resistance developing in S. Typhi isolates and other Gram-negative bacteria in endemic regions. The deployment of typhoid conjugate vaccines to control the disease in endemic regions may be the best defence.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests. AJP has previously conducted studies on behalf of Oxford University funded by vaccine manufacturers, but currently does not undertake industry funded clinical trials. AJP chairs the UK Department of Health’s (DH) Joint Committee on Vaccination and Immunisation (JCVI) and is a member of the World Health Organisation Strategic Group of Experts (SAGE); the views expressed in this manuscript do not necessarily reflect the views of JCVI, DH or SAGE. The other authors have no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

