Mice lacking RAP1 show early onset and higher rates of DEN-induced hepatocellular carcinomas in female mice
- PMID: 30307978
- PMCID: PMC6187989
- DOI: 10.1371/journal.pone.0204909
Mice lacking RAP1 show early onset and higher rates of DEN-induced hepatocellular carcinomas in female mice
Abstract
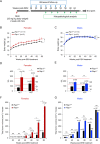
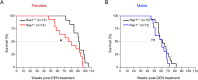
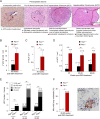
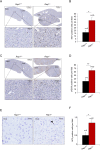
RAP1, a component of the telomere-protective shelterin complex, has been shown to have both telomeric and non-telomeric roles. In the liver, RAP1 is involved in the regulation of metabolic transcriptional programs. RAP1-deficient mice develop obesity and hepatic steatosis, these phenotypes being more severe in females than in males. As hepatic steatosis and obesity have been related to increased liver cancer in mice and humans, we set out to address whether RAP1 deficiency resulted in increased liver cancer upon chemical liver carcinogenesis. We found that Rap1-/- females were more susceptible to DEN-induced liver damage and hepatocellular carcinoma (HCC). DEN-treated Rap1-/- female livers showed an earlier onset of both premalignant and malignant liver lesions, which were characterized by increased abundance of γH2AX-positive cells, increased proliferation and shorter telomeres. These findings highlight an important role for RAP1 in protection from liver damage and liver cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

