Serum asunaprevir concentrations showing correlation with the extent of liver fibrosis as a factor inducing liver injuries in patients with genotype-1b hepatitis C virus receiving daclatasvir plus asunaprevir therapy
- PMID: 30308053
- PMCID: PMC6181393
- DOI: 10.1371/journal.pone.0205600
Serum asunaprevir concentrations showing correlation with the extent of liver fibrosis as a factor inducing liver injuries in patients with genotype-1b hepatitis C virus receiving daclatasvir plus asunaprevir therapy
Abstract
Aims: Liver injury can occur during antiviral therapies with direct-acting antivirals (DAAs), potentially necessitating discontinuation of the therapies, with consequent worsening of the sustained viral response (SVR) rates, in patients with hepatitis C virus (HCV). To clarify the mechanisms involved in serum transaminase level elevation, we performed a retrospective evaluation of the serum concentrations of daclatasvir and asunaprevir, both classified as DAAs, in patients receiving treatment with a combination of the two drugs.
Methods: Subjects were 278 Japanese patients with genotype-1b HCV who received daclatasvir plus asunaprevir therapy for more than 4 weeks. Serum concentrations of both the DAAs were measured at 4 weeks after the initiation of therapy.
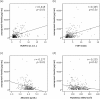
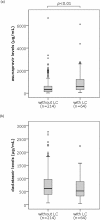
Result: Liver injuries including serum AST and/or ALT level elevation to 150 U/L or over were found in 34 patients (12.2%). Multivariate logistic regression analysis identified serum asunaprevir concentrations as being significantly associated with developing liver injury, with an odds ratio of 1.046 (95% confidence interval 1.011-1.082, p<0.05). Serum asunaprevir concentrations showed correlation with the extent of liver fibrosis, estimated by peripheral platelets counts and serum albumin levels and baseline and FIB4 index and serum Mac-2 binding protein glycosylation isomer (M2BPGi) levels at 4 weeks of the therapy; the concentrations were significantly higher among patients showing 3.0 or more of M2BPGi levels than among those with the levels less than 3.0; on the other hand, no such correlation/difference was found in serum daclatasvir concentrations.
Conclusion: High serum concentrations of serum asunaprevir, which were associated with the extent of liver fibrosis, appear to provoke the occurrence of liver injury in patients with genotype-1b HCV receiving combined daclatasvir plus asunaprevir therapy.
Conflict of interest statement
SM has received patent royalties from SRL Inc., has received speaking fees or hon-oraria from AbbVie GK, Ajinomoto Pharmaceuticals Co. Ltd., Bristol Myers Squibb Co., Gilead Sciences Inc., MSD K.K., Sumitomo Dainippon Pharma Co., Toray Medical Co. Ltd., has received research grants from A2 Healthcare Co., AbbVie GK, Bristol Myers Squibb Co., Chugai Pharma-ceutical Co. Ltd., EA Pharma Co. Ltd., Mitsubishi Tanabe Pharma Co., MSD K.K., Sumitomo Dainippon Pharma Co., Toray Medical Co. Ltd. YU has received research grants from AbbVie GK. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Uchida Y, Kouyama J-I, Naiki K, Mochida S. A novel simple assay system to quantify the percent HCV-RNA levels of NS5A Y93H mutant strains and Y93 wild-type strains relative to the total HCV-RNA levels to determine the indication for antiviral therapy with NS5A inhibitors. PLoS One. 2014. November 14;9(11):e112647 10.1371/journal.pone.0112647 - DOI - PMC - PubMed
-
- Uchida Y, Kouyama J-I, Naiki K, Sugawara K, Ando S, Nakao M, et al. Significance of variants associated with resistance to NS5A inhibitors in Japanese patients with genotype 1b hepatitis C virus infection as evaluated using cycling-probe real-time PCR combined with direct sequencing. J Gastroenterol. 2016. March;51(3):260–70. 10.1007/s00535-015-1106-8 - DOI - PubMed
-
- Uchida Y, Kouyama JI, Naiki K, Sugawara K, Inao M, Imai Y, et al. Development of rare resistance-associated variants that are extremely tolerant against NS5A inhibitors during daclatasvir/asunaprevir therapy by a two-hit mechanism. Hepatol Res. 2016. November;46(12):1234–1246. 10.1111/hepr.12673 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

