CD47 Protects Synapses from Excess Microglia-Mediated Pruning during Development
- PMID: 30308165
- PMCID: PMC6314207
- DOI: 10.1016/j.neuron.2018.09.017
CD47 Protects Synapses from Excess Microglia-Mediated Pruning during Development
Abstract
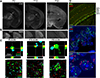
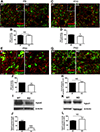
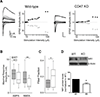
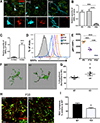
Microglia regulate synaptic circuit remodeling and phagocytose synaptic material in the healthy brain; however, the mechanisms directing microglia to engulf specific synapses and avoid others remain unknown. Here, we demonstrate that an innate immune signaling pathway protects synapses from inappropriate removal. The expression patterns of CD47 and its receptor, SIRPα, correlated with peak pruning in the developing retinogeniculate system, and mice lacking these proteins exhibited increased microglial engulfment of retinogeniculate inputs and reduced synapse numbers in the dorsal lateral geniculate nucleus. CD47-deficient mice also displayed increased functional pruning, as measured by electrophysiology. In addition, CD47 was found to be required for neuronal activity-mediated changes in engulfment, as microglia in CD47 knockout mice failed to display preferential engulfment of less active inputs. Taken together, these results demonstrate that CD47-SIRPα signaling prevents excess microglial phagocytosis and show that molecular brakes can be regulated by activity to protect specific inputs.
Keywords: CD47; SIRPα; activity; don’t eat me; microglia; phagocytosis; protective signal; pruning; refinement; retinogeniculate system.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests. Beth Stevens serves on the scientific advisory board of Annexon LLC. and is a minor shareholder of Annexon LLC but this is unrelated to the submitted work.
Figures



Comment in
-
A 'don't eat me' immune signal protects neuronal connections.Nature. 2018 Nov;563(7729):42-43. doi: 10.1038/d41586-018-07165-8. Nature. 2018. PMID: 30374080 No abstract available.
-
Stop, don't prune me!Nat Rev Neurosci. 2018 Dec;19(12):712-713. doi: 10.1038/s41583-018-0090-4. Nat Rev Neurosci. 2018. PMID: 30405176 No abstract available.
References
-
- Adams S, van der Laan LJW, Vernon-Wilson E, Renardel de Lavalette C, Döpp EA, Dijkstra CD, Simmons DL, and van den Berg TK (1998). Signal-Regulatory Protein Is Selectively Expressed by Myeloid and Neuronal Cells. The Journal of Immunology 161, 1853–1859. - PubMed
-
- Barria A, and Malinow R (2002). Subunit-specific NMDA receptor trafficking to synapses. Neuron 35, 345–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

