Descemet's Membrane Biomimetic Microtopography Differentiates Human Mesenchymal Stem Cells Into Corneal Endothelial-Like Cells
- PMID: 30308581
- PMCID: PMC6282677
- DOI: 10.1097/ICO.0000000000001765
Descemet's Membrane Biomimetic Microtopography Differentiates Human Mesenchymal Stem Cells Into Corneal Endothelial-Like Cells
Abstract
Purpose: Loss of corneal endothelial cells (CECs) bears disastrous consequences for the patient, including corneal clouding and blindness. Corneal transplantation is currently the only therapy for severe corneal disorders. However, the worldwide shortages of corneal donor material generate a strong demand for personalized stem cell-based alternative therapies. Because human mesenchymal stem cells are known to be sensitive to their mechanical environments, we investigated the mechanotransductive potential of Descemet membrane-like microtopography (DLT) to differentiate human mesenchymal stem cells into CEC-like cells.
Methods: Master molds with inverted DLT were produced by 2-photon lithography (2-PL). To measure the mechanotransductive potential of DLT, mesenchymal stem cells were cultivated on silicone or collagen imprints with DLT. Changes in morphology were imaged, and changes in gene expression of CEC typical genes such as zonula occludens (ZO-1), sodium/potassium (Na/K)-ATPase, paired-like homeodomain 2 (PITX2), and collagen 8 (COL-8) were measured with real-time polymerase chain reaction. At least immunofluorescence analysis has been conducted to confirm gene data on the protein level.
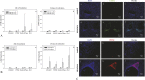
Results: Adhesion of MSCs to DLT molded in silicone and particularly in collagen initiates polygonal morphology and monolayer formation and enhances not only transcription of CEC typical genes such as ZO-1, Na/K-ATPase, PITX2, and COL-8 but also expression of the corresponding proteins.
Conclusions: Artificial reproduction of Descemet membrane with respect to topography and similar stiffness offers a potential innovative way to bioengineer a functional CEC monolayer from autologous stem cells.
Figures



References
-
- Engelmann K, Bednarz J, Valtink M. Prospects for endothelial transplantation. Exp Eye Res. 2004;78:573–578. - PubMed
-
- Joyce NC. Cell cycle status in human corneal endothelium. Exp Eye Res. 2005;81:629–638. - PubMed
-
- Tan DT, Dart JK, Holland EJ, et al. Corneal transplantation. Lancet. 2012;379:1749–1761. - PubMed
-
- Wu EI, Ritterband DC, Yu G, et al. Graft rejection following descemet stripping automated endothelial keratoplasty: features, risk factors, and outcomes. Am J Ophthalmol. 2012;153:949–957. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

