Induction of ATP Release, PPIX Transport, and Cholesterol Uptake by Human Red Blood Cells Using a New Family of TSPO Ligands
- PMID: 30308949
- PMCID: PMC6213633
- DOI: 10.3390/ijms19103098
Induction of ATP Release, PPIX Transport, and Cholesterol Uptake by Human Red Blood Cells Using a New Family of TSPO Ligands
Abstract
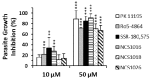
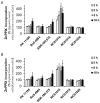
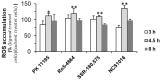
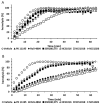
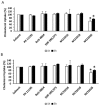
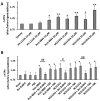
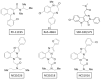
Two main isoforms of the Translocator Protein (TSPO) have been identified. TSPO1 is ubiquitous and is mainly present at the outer mitochondrial membrane of most eukaryotic cells, whereas, TSPO2 is specific to the erythroid lineage, located at the plasma membrane, the nucleus, and the endoplasmic reticulum. The design of specific tools is necessary to determine the molecular associations and functions of TSPO, which remain controversial nowadays. We recently demonstrated that TSPO2 is involved in a supramolecular complex of the erythrocyte membrane, where micromolar doses of the classical TSPO ligands induce ATP release and zinc protoporphyrin (ZnPPIX) transport. In this work, three newly-designed ligands (NCS1016, NCS1018, and NCS1026) were assessed for their ability to modulate the functions of various erythrocyte's and compare them to the TSPO classical ligands. The three new ligands were effective in reducing intraerythrocytic Plasmodium growth, without compromising erythrocyte survival. While NCS1016 and NCS1018 were the most effective ligands in delaying sorbitol-induced hemolysis, NCS1016 induced the highest uptake of ZnPPIX and NCS1026 was the only ligand inhibiting the cholesterol uptake. Differential effects of ligands are probably due, not only, to ligand features, but also to the dynamic interaction of TSPO with various partners at the cell membrane. Further studies are necessary to fully understand the mechanisms of the TSPO's complex activation.
Keywords: ATP; TSPO; TSPO2; VDAC; ZnPPIX; erythrocyte; malaria; plasmodium; red blood cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
TSPO ligands stimulate ZnPPIX transport and ROS accumulation leading to the inhibition of P. falciparum growth in human blood.Sci Rep. 2016 Sep 19;6:33516. doi: 10.1038/srep33516. Sci Rep. 2016. PMID: 27641616 Free PMC article.
-
Human erythrocytes release ATP by a novel pathway involving VDAC oligomerization independent of pannexin-1.Sci Rep. 2018 Jul 30;8(1):11384. doi: 10.1038/s41598-018-29885-7. Sci Rep. 2018. PMID: 30061676 Free PMC article.
-
Tetrapyrroles as Endogenous TSPO Ligands in Eukaryotes and Prokaryotes: Comparisons with Synthetic Ligands.Int J Mol Sci. 2016 Jun 4;17(6):880. doi: 10.3390/ijms17060880. Int J Mol Sci. 2016. PMID: 27271616 Free PMC article. Review.
-
The 18 kDa mitochondrial translocator protein (TSPO) prevents accumulation of protoporphyrin IX. Involvement of reactive oxygen species (ROS).Curr Mol Med. 2012 May;12(4):494-501. doi: 10.2174/1566524011207040494. Curr Mol Med. 2012. PMID: 22376065
-
Translocator protein: pharmacology and steroidogenesis.Biochem Soc Trans. 2015 Aug;43(4):572-8. doi: 10.1042/BST20150061. Epub 2015 Aug 3. Biochem Soc Trans. 2015. PMID: 26551695 Review.
Cited by
-
Downregulation of Mitochondrial TSPO Inhibits Mitophagy and Reduces Enucleation during Human Terminal Erythropoiesis.Int J Mol Sci. 2020 Nov 28;21(23):9066. doi: 10.3390/ijms21239066. Int J Mol Sci. 2020. PMID: 33260618 Free PMC article.
-
Imaging tumor and ascites-associated macrophages in a mouse model of metastatic ovarian cancer.EJNMMI Res. 2024 Nov 29;14(1):121. doi: 10.1186/s13550-024-01157-8. EJNMMI Res. 2024. PMID: 39612052 Free PMC article.
-
Effect of mitochondrial translocator protein TSPO on LPS-induced cardiac dysfunction.J Adv Res. 2025 Aug;74:455-469. doi: 10.1016/j.jare.2024.10.004. Epub 2024 Oct 9. J Adv Res. 2025. PMID: 39389308 Free PMC article.
-
[18F]LW223 has low non-displaceable binding in murine brain, enabling high sensitivity TSPO PET imaging.J Cereb Blood Flow Metab. 2024 Mar;44(3):397-406. doi: 10.1177/0271678X231205661. Epub 2023 Oct 5. J Cereb Blood Flow Metab. 2024. PMID: 37795635 Free PMC article.
-
Efficaciousness of Low Affinity Compared to High Affinity TSPO Ligands in the Inhibition of Hypoxic Mitochondrial Cellular Damage Induced by Cobalt Chloride in Human Lung H1299 Cells.Biomedicines. 2020 May 2;8(5):106. doi: 10.3390/biomedicines8050106. Biomedicines. 2020. PMID: 32370132 Free PMC article.
References
-
- Papadopoulos V., Baraldi M., Guilarte T.R., Knudsen T.B., Lacapere J.J., Lindemann P., Norenberg M.D., Nutt D., Weizman A., Zhang M.R., et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharm. Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. - DOI - PubMed
-
- Fan J., Lindemann P., Feuilloley M.G., Papadopoulos V. Structural and functional evolution of the translocator protein (18 kDa) Curr. Mol. Med. 2012;12:369–386. - PubMed
-
- Marginedas-Freixa I., Hattab C., Bouyer G., Halle F., Chene A., Lefevre S.D., Cambot M., Cueff A., Schmitt M., Gamain B., et al. TSPO ligands stimulate ZnPPIX transport and ROS accumulation leading to the inhibition of P. falciparum growth in human blood. Sci. Rep. 2016;6:33516. doi: 10.1038/srep33516. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

