Chemical Diversity of Metal Sulfide Minerals and Its Implications for the Origin of Life
- PMID: 30308967
- PMCID: PMC6316247
- DOI: 10.3390/life8040046
Chemical Diversity of Metal Sulfide Minerals and Its Implications for the Origin of Life
Abstract
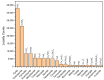
Prebiotic organic synthesis catalyzed by Earth-abundant metal sulfides is a key process for understanding the evolution of biochemistry from inorganic molecules, yet the catalytic functions of sulfides have remained poorly explored in the context of the origin of life. Past studies on prebiotic chemistry have mostly focused on a few types of metal sulfide catalysts, such as FeS or NiS, which form limited types of products with inferior activity and selectivity. To explore the potential of metal sulfides on catalyzing prebiotic chemical reactions, here, the chemical diversity (variations in chemical composition and phase structure) of 304 natural metal sulfide minerals in a mineralogy database was surveyed. Approaches to rationally predict the catalytic functions of metal sulfides are discussed based on advanced theories and analytical tools of electrocatalysis such as proton-coupled electron transfer, structural comparisons between enzymes and minerals, and in situ spectroscopy. To this end, we introduce a model of geoelectrochemistry driven prebiotic synthesis for chemical evolution, as it helps us to predict kinetics and selectivity of targeted prebiotic chemistry under "chemically messy conditions". We expect that combining the data-mining of mineral databases with experimental methods, theories, and machine-learning approaches developed in the field of electrocatalysis will facilitate the prediction and verification of catalytic performance under a wide range of pH and Eh conditions, and will aid in the rational screening of mineral catalysts involved in the origin of life.
Keywords: density functional theory; electrocatalysis; mineral catalysis; mineral diversity; origin of life; prebiotic chemistry; sulfide minerals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kitadai N., Maruyama S. Origins of building blocks of life: A review. Geosci. Front. 2018;9:1117–1153. doi: 10.1016/j.gsf.2017.07.007. - DOI
-
- Cody G.D. Geochemical Connections to Primitive Metabolism. Elements. 2005;1:139–143. doi: 10.2113/gselements.1.3.139. - DOI
-
- Russell M.J., Hall A.J., Mellersh A.R. Natural and Laboratory-Simulated Thermal Geochemical Processes. Springer; Dordrecht, The Netherlands: 2003. On the Dissipation of Thermal and Chemical Energies on the Early Earth; pp. 325–388.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

