A Solvothermal Synthesis of TiO₂ Nanoparticles in a Non-Polar Medium to Prepare Highly Stable Nanofluids with Improved Thermal Properties
- PMID: 30309047
- PMCID: PMC6215110
- DOI: 10.3390/nano8100816
A Solvothermal Synthesis of TiO₂ Nanoparticles in a Non-Polar Medium to Prepare Highly Stable Nanofluids with Improved Thermal Properties
Abstract
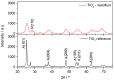
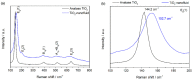
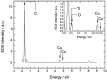
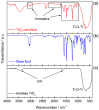
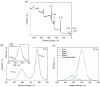
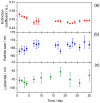
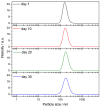
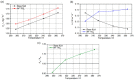
Nanofluids are systems with several interesting heat transfer applications, but it can be a challenge to obtain highly stable suspensions. One way to overcome this challenge is to create the appropriate conditions to disperse the nanomaterial in the fluid. However, when the heat transfer fluid used is a non-polar organic oil, there are complications due to the low polarity of this solvent. Therefore, this study introduces a method to synthesize TiO₂ nanoparticles inside a non-polar fluid typically used in heat transfer applications. Nanoparticles produced were characterized for their structural and chemical properties using techniques such as X-ray Diffraction (XRD), Raman spectroscopy, Transmission Electron Microscopy (TEM), Fourier Transform Infrared (FTIR) spectroscopy, and X-ray photoelectron spectroscopy (XPS). The nanofluid showed a high stability, which was analyzed by means of UV-vis spectroscopy and by measuring its particle size and ζ potential. So, this nanofluid will have many possible applications. In this work, the use as heat transfer fluid was tested. In this sense, nanofluid also presented enhanced isobaric specific heat and thermal conductivity values with regard to the base fluid, which led to the heat transfer coefficient increasing by 14.4%. Thus, the nanofluid prepared could be a promising alternative to typical HTFs thanks to its improved thermal properties and high stability resulting from the synthesis procedure.
Keywords: concentrating solar power; heat transfer process; nanofluids; nanoparticles; thermal properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Investigation on Synthesis, Stability, and Thermal Conductivity Properties of Water-Based SnO₂/Reduced Graphene Oxide Nanofluids.Materials (Basel). 2017 Dec 27;11(1):38. doi: 10.3390/ma11010038. Materials (Basel). 2017. PMID: 29280972 Free PMC article.
-
Two-Dimensional Tungsten Disulfide-Based Ethylene Glycol Nanofluids: Stability, Thermal Conductivity, and Rheological Properties.Nanomaterials (Basel). 2020 Jul 9;10(7):1340. doi: 10.3390/nano10071340. Nanomaterials (Basel). 2020. PMID: 32659972 Free PMC article.
-
A Novel Experimental Study on the Rheological Properties and Thermal Conductivity of Halloysite Nanofluids.Nanomaterials (Basel). 2020 Sep 14;10(9):1834. doi: 10.3390/nano10091834. Nanomaterials (Basel). 2020. PMID: 32937934 Free PMC article.
-
A critical review on thermal conductivity enhancement of graphene-based nanofluids.Adv Colloid Interface Sci. 2021 Aug;294:102452. doi: 10.1016/j.cis.2021.102452. Epub 2021 May 26. Adv Colloid Interface Sci. 2021. PMID: 34139659 Review.
-
Review on Nanofluids: Preparation, Properties, Stability, and Thermal Performance Augmentation in Heat Transfer Applications.ACS Omega. 2024 Jul 15;9(30):32328-32349. doi: 10.1021/acsomega.4c03279. eCollection 2024 Jul 30. ACS Omega. 2024. PMID: 39100289 Free PMC article. Review.
Cited by
-
Nanoparticle Synthesis and Their Integration into Polymer-Based Fibers for Biomedical Applications.Biomedicines. 2023 Jun 29;11(7):1862. doi: 10.3390/biomedicines11071862. Biomedicines. 2023. PMID: 37509502 Free PMC article. Review.
-
Impact of the Precursor on the Physicochemical Properties and Photoactivity of TiO2 Nanoparticles Produced in Supercritical CO2.Nanomaterials (Basel). 2023 Aug 13;13(16):2328. doi: 10.3390/nano13162328. Nanomaterials (Basel). 2023. PMID: 37630913 Free PMC article.
References
-
- Choi S.U.S. Enhancing thermal conductivity of fluids with nanoparticles. ASME-Publ.-Fed. 1995;231:99–106.
-
- Colangelo G., Favale E., Milanese M., de Risi A., Laforgia D. Cooling of electronic devices: Nanofluids contribution. Appl. Therm. Eng. 2017;127:421–435. doi: 10.1016/j.applthermaleng.2017.08.042. - DOI
-
- Asadi M., Asadi A., Aberoumand S. An experimental and theoretical investigation on the effects of adding hybrid nanoparticles on heat transfer efficiency and pumping power of an oil-based nanofluid as a coolant fluid. Int. J. Refrig. 2018;89:83–92. doi: 10.1016/j.ijrefrig.2018.03.014. - DOI
-
- Colangelo G., Favale E., Miglieta P., Milanese M., de Risi A. Thermal conductivity, viscosity and stability of Al3O3-diathermic oil nanofluids for solar energy systems. Energy. 2016;95:124–136. doi: 10.1016/j.energy.2015.11.032. - DOI
-
- Águila V.B., Vasco D.A., Galvez P.P., Zapata P.A. Effect of temperature and CuO-nanoparticle concentration on the thermal conductivity and viscosity of an organic phase-change material. Int. J. Heat Mass Transf. 2018;120:1009–1019. doi: 10.1016/j.ijheatmasstransfer.2017.12.106. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

