Single nucleotide polymorphism of <italic>GSTP1</italic> and pathological complete response in locally advanced rectal cancer patients treated with neoadjuvant concomitant radiochemotherapy
- PMID: 30309213
- PMCID: PMC6226144
- DOI: 10.3857/roj.2018.00094
Single nucleotide polymorphism of <italic>GSTP1</italic> and pathological complete response in locally advanced rectal cancer patients treated with neoadjuvant concomitant radiochemotherapy
Abstract
Purpose: Standard treatment for locally advanced rectal cancer consists of neoadjuvant radiochemotherapy with concomitant fluoropyrimidine or oxaliplatin and surgery with curative intent. Pathological complete response has shown to be predictive for better outcome and survival; nevertheless there are no biological or genetic factors predictive for response to treatment. We explored the correlation between the single nucleotide polymorphisms (SNPs) GSTP1 (A313G) and XRCC1 (G28152A), and the pathological complete response and survival after neoadjuvant radiochemotherapy in locally advanced rectal cancer patients.
Materials and methods: Genotypes GSTP1 (A313G) and XRCC1 (G28152A) were determined by pyrosequencing technology in 80 patients affected by locally advanced rectal cancer.
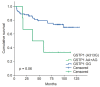
Results: The overall rate of pathological complete response in our study population was 18.75%. Patients homozygous AA for GSTP1 (A313G) presented a rate of pathological complete response of 26.6% as compared to 8.5% of the AG+GG population (p = 0.04). The heterozygous comparison (AA vs. AG) showed a significant difference in the rate of pathological complete response (26.6% vs. 6.8%; p = 0.034). GSTP1 AA+AG patients presented a 5- and 8-year cancer-specific survival longer than GSTP1 GG patients (87.7% and 83.3% vs. 44.4% and 44.4%, respectively) (p = 0.014). Overall survival showed only a trend toward significance in favor of the haplotypes GSTP1 AA+AG. No significant correlations were found for XRCC1 (G28152A).
Conclusion: Our results suggest that GSTP1 (A313G) may predict a higher rate of pathological complete response after neoadjuvant radiochemotherapy and a better outcome, and should be considered in a more extensive analysis with the aim of personalization of radiation treatment.
Keywords: Genetic marker; Prognostic factor; Radiochemotherapy; Rectal cancer; Single nucleotide polymorphism; Tumor response.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


Similar articles
-
Potential Role of Single Nucleotide Polymorphisms of XRCC1, XRCC3, and RAD51 in Predicting Acute Toxicity in Rectal Cancer Patients Treated With Preoperative Radiochemotherapy.Am J Clin Oncol. 2017 Dec;40(6):535-542. doi: 10.1097/COC.0000000000000182. Am J Clin Oncol. 2017. PMID: 25811296
-
Association of GSTP1 and XRCC1 gene polymorphisms with clinical outcome of advanced non-small cell lung cancer patients with cisplatin-based chemotherapy.Int J Clin Exp Pathol. 2015 Apr 1;8(4):4113-9. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26097600 Free PMC article.
-
XRCC1 gene polymorphism for prediction of response and prognosis in the multimodality therapy of patients with locally advanced rectal cancer.J Surg Res. 2010 Nov;164(1):e61-6. doi: 10.1016/j.jss.2010.08.002. Epub 2010 Sep 16. J Surg Res. 2010. PMID: 20863523
-
XRCC1 Arg399Gln and clinical outcome of platinum-based treatment for advanced non-small cell lung cancer: a meta-analysis in 17 studies.J Zhejiang Univ Sci B. 2012 Nov;13(11):875-83. doi: 10.1631/jzus.B1200083. J Zhejiang Univ Sci B. 2012. PMID: 23125080 Free PMC article. Review.
-
Is It Possible a Conservative Approach After Radiochemotherapy in Locally Advanced Rectal Cancer (LARC)? A Systematic Review of the Literature and Meta-analysis.J Gastrointest Cancer. 2019 Mar;50(1):98-108. doi: 10.1007/s12029-017-0041-8. J Gastrointest Cancer. 2019. PMID: 29273921
Cited by
-
Germline and Somatic Pharmacogenomics to Refine Rectal Cancer Patients Selection for Neo-Adjuvant Chemoradiotherapy.Front Pharmacol. 2020 Jun 17;11:897. doi: 10.3389/fphar.2020.00897. eCollection 2020. Front Pharmacol. 2020. PMID: 32625092 Free PMC article. Review.
-
Significant Association Between XRCC1 Expression and Its rs25487 Polymorphism and Radiotherapy-Related Cancer Prognosis.Front Oncol. 2021 May 19;11:654784. doi: 10.3389/fonc.2021.654784. eCollection 2021. Front Oncol. 2021. PMID: 34094945 Free PMC article.
-
Outcomes of Radiotherapy for Mesenchymal and Non-Mesenchymal Subtypes of Gastric Cancer.Cancers (Basel). 2020 Apr 10;12(4):943. doi: 10.3390/cancers12040943. Cancers (Basel). 2020. PMID: 32290335 Free PMC article.
-
Investigation of Genetic Polymorphisms Related GSTM1, GSTT1, GSTP1 Genes and their Association with Radiotherapy Toxicity among Head and Neck Cancer Patients.Asian Pac J Cancer Prev. 2025 Jan 1;26(1):49-57. doi: 10.31557/APJCP.2025.26.1.49. Asian Pac J Cancer Prev. 2025. PMID: 39873985 Free PMC article.
References
-
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. - PubMed
-
- Janjan NA, Khoo VS, Abbruzzese J, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44:1027–38. - PubMed
-
- Kim JS, Kim JS, Cho MJ, Song KS, Yoon WH. Preoperative chemoradiation using oral capecitabine in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2002;54:403–8. - PubMed
-
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–9. - PubMed
-
- Rodel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–89. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

