Gold nanoparticle distribution in advanced in vitro and ex vivo human placental barrier models
- PMID: 30309365
- PMCID: PMC6180500
- DOI: 10.1186/s12951-018-0406-6
Gold nanoparticle distribution in advanced in vitro and ex vivo human placental barrier models
Abstract
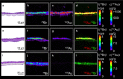
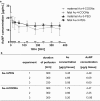
Background: Gold nanoparticles (AuNPs) are promising candidates to design the next generation NP-based drug formulations specifically treating maternal, fetal or placental complications with reduced side effects. Profound knowledge on AuNP distribution and effects at the human placental barrier in dependence on the particle properties and surface modifications, however, is currently lacking. Moreover, the predictive value of human placental transfer models for NP translocation studies is not yet clearly understood, in particular with regards to differences between static and dynamic exposures. To understand if small (3-4 nm) AuNPs with different surface modifications (PEGylated versus carboxylated) are taken up and cross the human placental barrier, we performed translocation studies in a static human in vitro co-culture placenta model and the dynamic human ex vivo placental perfusion model. The samples were analysed using ICP-MS, laser ablation-ICP-MS and TEM analysis for sensitive, label-free detection of AuNPs.
Results: After 24 h of exposure, both AuNP types crossed the human placental barrier in vitro, although in low amounts. Even though cellular uptake was higher for carboxylated AuNPs, translocation was slightly increased for PEGylated AuNPs. After 6 h of perfusion, only PEGylated AuNPs were observed in the fetal circulation and tissue accumulation was similar for both AuNP types. While PEGylated AuNPs were highly stable in the biological media and provided consistent results among the two placenta models, carboxylated AuNPs agglomerated and adhered to the perfusion device, resulting in different cellular doses under static and dynamic exposure conditions.
Conclusions: Gold nanoparticles cross the human placental barrier in limited amounts and accumulate in placental tissue, depending on their size- and/or surface modification. However, it is challenging to identify the contribution of individual characteristics since they often affect colloidal particle stability, resulting in different biological interaction in particular under static versus dynamic conditions. This study highlights that human ex vivo and in vitro placenta models can provide valuable mechanistic insights on NP uptake and translocation if accounting for NP stability and non-specific interactions with the test system.
Keywords: Ex vivo placenta perfusion; Gold nanoparticle; Nanoparticle agglomeration; Placental in vitro co-culture model; Placental uptake and translocation.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

