The addition of chemotherapy in the definitive management of high risk prostate cancer
- PMID: 30309766
- PMCID: PMC6214780
- DOI: 10.1016/j.urolonc.2018.07.020
The addition of chemotherapy in the definitive management of high risk prostate cancer
Abstract
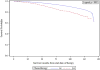
In attempt to improve long-term disease control outcomes for high-risk prostate cancer, numerous clinical trials have tested the addition of chemotherapy (CTX)-either adjuvant or neoadjuvant-to definitive local therapy, either radical prostatectomy (RP) or radiation therapy (RT). Neoadjuvant trials generally confirm safety, feasibility, and pre-RP PSA reduction, but rates of pathologic complete response are rare, and no indications for neoadjuvant CTX have been firmly established. Adjuvant regimens have included CTX alone or in combination with androgen deprivation therapy (ADT). Here we provide a review of the relevant literature, and also quantify utilization of CTX in the definitive management of localized high-risk prostate cancer by querying the National Cancer Data Base. Between 2004 and 2013, 177 patients (of 29,659 total) treated with definitive RT, and 995 (of 367,570 total) treated with RP had CTX incorporated into their treatment regimens. Low numbers of RT + CTX patients precluded further analysis of this population, but we investigated the impact of CTX on overall survival (OS) for patients treated with RP +/- CTX. Disease-free survival or biochemical-recurrence-free survival are not available through the National Cancer Data Base. Propensity-score matching was conducted as patients treated with CTX were a higher-risk group. For nonmatched groups, OS at 5-years was 89.6% for the CTX group vs. 95.6%, for the no-CTX group (P < 0.01). The difference in OS between CTX and no-CTX groups did not persist after propensity-score matching, with 5-year OS 89.6% vs. 90.9%, respectively (Hazard ratio 0.99; P = 0.88). In summary, CTX was not shown to improve OS in this retrospective study. Multimodal regimens-such as RP followed by ADT, RT, and CTX; or RT in conjunction with ADT followed by CTX-have shown promise, but long-term follow-up of randomized data is required.
Keywords: ADT, Androgen deprivation therapy; AJCC, American Joint Committee on Cancer; Abbreviations: CTX, Chemotherapy; Adjuvant; CI, Confidence interval; Chemotherapy; CoC, Commission on Cancer; HR, Hazard ratio; High-risk prostate cancer; MVA, Multivariable analysis; NCDB, National Cancer Data Base; Neoadjuvant; OS, Overall survival; PSA, Prostate-specific antigen; PSM, Propensity score matching; Prostatectomy; RP, Radical prostatectomy; RT, Radiation therapy; Radiation therapy; UVA, Univariate analysis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- Clark PE, Peereboom DM, Dreicer R, Levin HS, Clark SB, Klein EA. Phase II trial of neoadjuvant estramustine and etoposide plus radical prostatectomy for locally advanced prostate cancer. Urology. 2001;57:281–5. - PubMed
-
- Dreicer R, Magi-Galluzzi C, Zhou M, et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology. 2004;63:1138–42. - PubMed
-
- Febbo PG, Richie JP, George DJ, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–40. - PubMed
-
- Magi-Galluzzi C, Zhou M, Reuther AM, Dreicer R, Klein EA. Neoadjuvant docetaxel treatment for locally advanced prostate cancer: a clinicopathologic study. Cancer. 2007;110:1248–54. - PubMed
-
- Oh WK, George DJ, Kaufman DS, et al. Neoadjuvant docetaxel followed by radical prostatectomy in patients with high-risk localized prostate cancer: a preliminary report. Semin Oncol. 2001;28:40–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

