Clinical and Regulatory Considerations for Central Venous Catheters for Hemodialysis
- PMID: 30309840
- PMCID: PMC6302318
- DOI: 10.2215/CJN.14251217
Clinical and Regulatory Considerations for Central Venous Catheters for Hemodialysis
Abstract
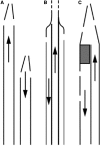
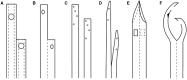
Central venous catheters remain a vital option for access for patients receiving maintenance hemodialysis. There are many important and evolving clinical and regulatory considerations for all stakeholders for these devices. Innovation and transparent and comprehensive regulatory review of these devices is essential to stimulate innovation to help promote better outcomes for patients receiving maintenance hemodialysis. A workgroup that included representatives from academia, industry, and the US Food and Drug Administration was convened to identify the major design considerations and clinical and regulatory challenges of central venous catheters for hemodialysis. Our intent is to foster improved understanding of these devices and provide the foundation for strategies to foster innovation of these devices.
Keywords: chronic dialysis; dialysis access; end stage kidney disease; hemodialysis; hemodialysis access.
Copyright © 2018 by the American Society of Nephrology.
Figures
References
-
- USRDS: 2015 USRDS annual data report, volume 2: End-stage renal disease. Available at: https://www.usrds.org/2015/download/vol2_USRDS_ESRD_15. Accessed August 1, 2016
-
- Ash SR: History of Central Venous Access for Dialysis, Interventional Nephrology Self-Assessment Program Phase 2. ASDIN, 2016
-
- Shaldon S, Chiandussi L, Higgs B: Hemodialysis by percutaneous catheterization of the femoral artery and vein with regional heparinization. Lancet 2: 857–859, 1961
-
- Uldall PR, Woods F, Merchant N, Crichton E, Carter H: A double-lumen subclavian cannula (DLSC) for temporary hemodialysis access. Trans Am Soc Artif Intern Organs 26: 93–98, 1980 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

