Polyreactive IgM initiates complement activation by PF4/heparin complexes through the classical pathway
- PMID: 30309891
- PMCID: PMC6284214
- DOI: 10.1182/blood-2018-03-834598
Polyreactive IgM initiates complement activation by PF4/heparin complexes through the classical pathway
Abstract
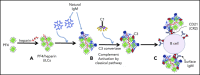
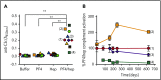
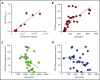
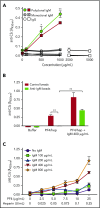
The mechanisms by which exposure to heparin initiates antibody responses in many, if not most, recipients are poorly understood. We recently demonstrated that antigenic platelet factor 4 (PF4)/heparin complexes activate complement in plasma and bind to B cells. Here, we describe how this process is initiated. We observed wide stable variation in complement activation when PF4/heparin was added to plasma of healthy donors, indicating a responder "phenotype" (high, intermediate, or low). Proteomic analysis of plasma from these healthy donors showed a strong correlation between complement activation and plasma immunoglobulin M (IgM) levels (r = 0.898; P < .005), but not other Ig isotypes. Complement activation response to PF4/heparin in plasma displaying the low donor phenotype was enhanced by adding pooled IgM from healthy donors, but not monoclonal IgM. Depletion of IgM from plasma abrogated C3c generation by PF4/heparin. The complement-activating features of IgM are likely mediated by nonimmune, or natural, IgM, as cord blood and a monoclonal polyreactive IgM generate C3c in the presence of PF4/heparin. IgM facilitates complement and antigen deposition on B cells in vitro and in patients receiving heparin. Anti-C1q antibody prevents IgM-mediated complement activation by PF4/heparin complexes, indicating classical pathway involvement. These studies demonstrate that variability in plasma IgM levels correlates with functional complement responses to PF4/heparin. Polyreactive IgM binds PF4/heparin, triggers activation of the classical complement pathway, and promotes antigen and complement deposition on B cells. These studies provide new insights into the evolution of the heparin-induced thrombocytopenia immune response and may provide a biomarker of risk.
Conflict of interest statement
Conflict-of-interest disclosure: G.M.A. has an awarded patent for KKO (US Application NO 60/143,536); G.M.A., M.P., and D.B.C. have pending intellectual property applications. The remaining authors declare no competing financial interests.
Figures

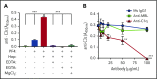
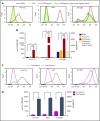
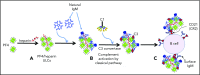
Comment in
-
Natural, not immune; classical, not alternative.Blood. 2018 Dec 6;132(23):2421-2422. doi: 10.1182/blood-2018-10-880799. Blood. 2018. PMID: 30523121 No abstract available.
Similar articles
-
Pathogenicity of IgA and/or IgM antibodies to heparin-PF4 complexes in patients with heparin-induced thrombocytopenia.Br J Haematol. 1996 Mar;92(4):954-9. doi: 10.1046/j.1365-2141.1996.407945.x. Br J Haematol. 1996. PMID: 8616093
-
The antigenic complex in HIT binds to B cells via complement and complement receptor 2 (CD21).Blood. 2016 Oct 6;128(14):1789-1799. doi: 10.1182/blood-2016-04-709634. Epub 2016 Jul 13. Blood. 2016. PMID: 27412887 Free PMC article.
-
Further insights into the anti-PF4/heparin IgM immune response.Thromb Haemost. 2016 Apr;115(4):752-61. doi: 10.1160/TH15-08-0654. Epub 2015 Oct 15. Thromb Haemost. 2016. PMID: 26467272
-
Evolving concepts of pathogenesis of heparin-induced thrombocytopenia: Diagnostic and therapeutic implications.Int J Lab Hematol. 2020 Jun;42 Suppl 1:25-32. doi: 10.1111/ijlh.13223. Int J Lab Hematol. 2020. PMID: 32543064 Review.
-
[Platelet factor 4, target of anti-heparin antibodies: application to biological diagnosis of heparin-induced thrombopenia].Ann Med Interne (Paris). 1997;148(2):142-9. Ann Med Interne (Paris). 1997. PMID: 9238439 Review. French.
Cited by
-
Ex vivo observation of granulocyte activity during thrombus formation.BMC Biol. 2022 Feb 7;20(1):32. doi: 10.1186/s12915-022-01238-x. BMC Biol. 2022. PMID: 35125118 Free PMC article.
-
Engineered IgM and IgG cleaving enzymes for mitigating antibody neutralization and complement activation in AAV gene transfer.Mol Ther. 2024 Jul 3;32(7):2080-2093. doi: 10.1016/j.ymthe.2024.05.004. Epub 2024 May 7. Mol Ther. 2024. PMID: 38715362 Free PMC article.
-
Complement activation as a biomarker for platelet-activating antibodies in heparin-induced thrombocytopenia.J Thromb Haemost. 2025 Mar;23(3):1066-1076. doi: 10.1016/j.jtha.2024.12.015. Epub 2024 Dec 25. J Thromb Haemost. 2025. PMID: 39725085
-
Thrombotic anti-PF4 immune disorders: HIT, VITT, and beyond.Hematology Am Soc Hematol Educ Program. 2023 Dec 8;2023(1):1-10. doi: 10.1182/hematology.2023000503. Hematology Am Soc Hematol Educ Program. 2023. PMID: 38066843 Free PMC article.
-
Autoimmunity elicited by the chemokine response to adenovirus vector vaccines may underlie vaccine-induced immune thrombotic thrombocytopaenia: a hypothesis.Clin Transl Immunology. 2021 Oct 14;10(10):e1349. doi: 10.1002/cti2.1349. eCollection 2021. Clin Transl Immunology. 2021. PMID: 34691454 Free PMC article. No abstract available.
References
-
- Trossaërt M, Gaillard A, Commin PL, Amiral J, Vissac AM, Fressinaud E. High incidence of anti-heparin/platelet factor 4 antibodies after cardiopulmonary bypass surgery. Br J Haematol. 1998;101(4):653-655. - PubMed
-
- Pouplard C, May MA, Iochmann S, et al. . Antibodies to platelet factor 4-heparin after cardiopulmonary bypass in patients anticoagulated with unfractionated heparin or a low-molecular-weight heparin: clinical implications for heparin-induced thrombocytopenia. Circulation. 1999;99(19):2530-2536. - PubMed
-
- Bakchoul T, Zöllner H, Amiral J, et al. . Anti-protamine-heparin antibodies: incidence, clinical relevance, and pathogenesis. Blood. 2013;121(15):2821-2827. - PubMed
-
- Pouplard C, Leroux D, Rollin J, Amiral J, May MA, Gruel Y. Incidence of antibodies to protamine sulfate/heparin complexes incardiac surgery patients and impact on platelet activation and clinical outcome. Thromb Haemost. 2013;109(6):1141-1147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

