A Ycf2-FtsHi Heteromeric AAA-ATPase Complex Is Required for Chloroplast Protein Import
- PMID: 30309901
- PMCID: PMC6305978
- DOI: 10.1105/tpc.18.00357
A Ycf2-FtsHi Heteromeric AAA-ATPase Complex Is Required for Chloroplast Protein Import
Abstract
Chloroplasts import thousands of nucleus-encoded preproteins synthesized in the cytosol through the TOC and TIC translocons on the outer and inner envelope membranes, respectively. Preprotein translocation across the inner membrane requires ATP; however, the import motor has remained unclear. Here, we report that a 2-MD heteromeric AAA-ATPase complex associates with the TIC complex and functions as the import motor, directly interacting with various translocating preproteins. This 2-MD complex consists of a protein encoded by the previously enigmatic chloroplast gene ycf2 and five related nuclear-encoded FtsH-like proteins, namely, FtsHi1, FtsHi2, FtsHi4, FtsHi5, and FtsH12. These components are each essential for plant viability and retain the AAA-type ATPase domain, but only FtsH12 contains the zinc binding active site generally conserved among FtsH-type metalloproteases. Furthermore, even the FtsH12 zinc binding site is dispensable for its essential function. Phylogenetic analyses suggest that all AAA-type members of the Ycf2/FtsHi complex including Ycf2 evolved from the chloroplast-encoded membrane-bound AAA-protease FtsH of the ancestral endosymbiont. The Ycf2/FtsHi complex also contains an NAD-malate dehydrogenase, a proposed key enzyme for ATP production in chloroplasts in darkness or in nonphotosynthetic plastids. These findings advance our understanding of this ATP-driven protein translocation system that is unique to the green lineage of photosynthetic eukaryotes.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures
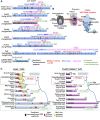

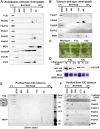
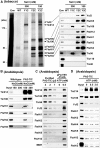

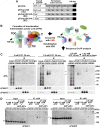



Comment in
-
A Force-Generating Machine in the Plant's Powerhouse: A Pulling AAA-ATPase Motor Drives Protein Translocation into Chloroplasts.Plant Cell. 2018 Nov;30(11):2646-2647. doi: 10.1105/tpc.18.00751. Epub 2018 Oct 11. Plant Cell. 2018. PMID: 30309903 Free PMC article. No abstract available.
-
Protein Import Motors in Chloroplasts: On the Role of Chaperones.Plant Cell. 2020 Mar;32(3):536-542. doi: 10.1105/tpc.19.00300. Epub 2020 Jan 13. Plant Cell. 2020. PMID: 31932485 Free PMC article. No abstract available.
-
Reply: The Revised Model for Chloroplast Protein Import.Plant Cell. 2020 Mar;32(3):543-546. doi: 10.1105/tpc.19.00821. Epub 2020 Jan 14. Plant Cell. 2020. PMID: 31937598 Free PMC article. No abstract available.
References
-
- Boudreau E., Turmel M., Goldschmidt-Clermont M., Rochaix J.-D., Sivan S., Michaels A., Leu S. (1997). A large open reading frame (orf1995) in the chloroplast DNA of Chlamydomonas reinhardtii encodes an essential protein. Mol. Gen. Genet. 253: 649–653. - PubMed
-
- Bruce B.D., Perry S., Froehlich J., Keegstra K. (1994). In vitro Import of Proteins into Chloroplasts. In Plant Molecular Biology Manual, Gelvin S.B., Schilperoot R.A., eds (Dordrecht: Springer; ), pp. 1–15.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

