Ultrafast epithelial contractions provide insights into contraction speed limits and tissue integrity
- PMID: 30309963
- PMCID: PMC6217427
- DOI: 10.1073/pnas.1802934115
Ultrafast epithelial contractions provide insights into contraction speed limits and tissue integrity
Abstract
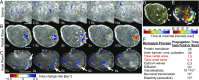
By definition of multicellularity, all animals need to keep their cells attached and intact, despite internal and external forces. Cohesion between epithelial cells provides this key feature. To better understand fundamental limits of this cohesion, we study the epithelium mechanics of an ultrathin (∼25 μm) primitive marine animal Trichoplax adhaerens, composed essentially of two flat epithelial layers. With no known extracellular matrix and no nerves or muscles, T. adhaerens has been claimed to be the "simplest known living animal," yet is still capable of coordinated locomotion and behavior. Here we report the discovery of the fastest epithelial cellular contractions known in any metazoan, to be found in T. adhaerens dorsal epithelium (50% shrinkage of apical cell area within one second, at least an order of magnitude faster than other known examples). Live imaging reveals emergent contractile patterns that are mostly sporadic single-cell events, but also include propagating contraction waves across the tissue. We show that cell contraction speed can be explained by current models of nonmuscle actin-myosin bundles without load, while the tissue architecture and unique mechanical properties are softening the tissue, minimizing the load on a contracting cell. We propose a hypothesis, in which the physiological role of the contraction dynamics is to resist external stresses while avoiding tissue rupture ("active cohesion"), a concept that can be further applied to engineering of active materials.
Keywords: biomechanics; cell contractility; epithelium; metazoan evolution; tissue integrity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–1342. - PubMed
-
- Maître J-L, Niwayama R, Turlier H, Nédélec F, Hiiragi T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat Cell Biol. 2015;17:849–855. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

