K+ Efflux Antiporters 4, 5, and 6 Mediate pH and K+ Homeostasis in Endomembrane Compartments
- PMID: 30309966
- PMCID: PMC6288736
- DOI: 10.1104/pp.18.01053
K+ Efflux Antiporters 4, 5, and 6 Mediate pH and K+ Homeostasis in Endomembrane Compartments
Abstract
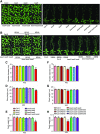
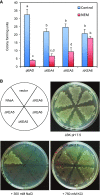
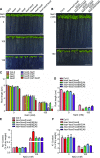
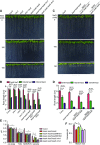
KEA4, KEA5, and KEA6 are members of the Arabidopsis (Arabidopsis thaliana) K+ efflux antiporter (KEA) family that share high sequence similarity but whose function remains unknown. Here, we show their gene expression pattern, subcellular localization, and physiological function in Arabidopsis. KEA4, KEA5, and KEA6 had similar tissue expression patterns, and the three KEA proteins localized to the Golgi, the trans-Golgi network, and the prevacuolar compartment/multivesicular bodies, suggesting overlapping roles of these proteins in the endomembrane system. Phenotypic analyses of single, double, and triple mutants confirmed functional redundancy. The triple mutant kea4 kea5 kea6 had small rosettes, short seedlings, and was sensitive to low K+ availability and to the sodicity imposed by high salinity. Also, the kea4 kea5 kea6 mutant plants had a reduced luminal pH in the Golgi, trans-Golgi network, prevacuolar compartment, and vacuole, in accordance with the K/H exchange activity of KEA proteins. Genetic analysis indicated that KEA4, KEA5, and KEA6 as well as endosomal Na+/H+exchanger5 (NHX5) and NHX6 acted coordinately to facilitate endosomal pH homeostasis and salt tolerance. Neither cancelling nor overexpressing the vacuolar antiporters NHX1 and NHX2 in the kea4 kea5 kea6 mutant background altered the salt-sensitive phenotype. The NHX1 and NHX2 proteins in the kea4 kea5 kea6 mutant background could not suppress the acidity of the endomembrane system but brought the vacuolar pH close to wild-type values. Together, these data signify that KEA4, KEA5, and KEA6 are endosomal K+ transporters functioning in maintaining pH and ion homeostasis in the endomembrane network.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures






References
-
- Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285: 1256–1258 - PubMed
-
- Apse MP, Sottosanto JB, Blumwald E (2003) Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J 36: 229–239 - PubMed
-
- Aranda-Sicilia MN, Cagnac O, Chanroj S, Sze H, Rodríguez-Rosales MP, Venema K (2012) Arabidopsis KEA2, a homolog of bacterial KefC, encodes a K+/H+ antiporter with a chloroplast transit peptide. Biochim Biophys Acta 1818: 2362–2371 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

