Rifampicin can induce antibiotic tolerance in mycobacteria via paradoxical changes in rpoB transcription
- PMID: 30310059
- PMCID: PMC6181997
- DOI: 10.1038/s41467-018-06667-3
Rifampicin can induce antibiotic tolerance in mycobacteria via paradoxical changes in rpoB transcription
Abstract
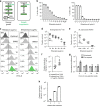
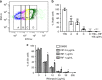
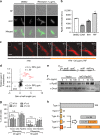
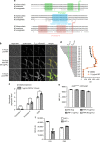
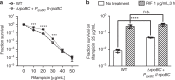
Metrics commonly used to describe antibiotic efficacy rely on measurements performed on bacterial populations. However, certain cells in a bacterial population can continue to grow and divide, even at antibiotic concentrations that kill the majority of cells, in a phenomenon known as antibiotic tolerance. Here, we describe a form of semi-heritable tolerance to the key anti-mycobacterial agent rifampicin, which is known to inhibit transcription by targeting the β subunit of the RNA polymerase (RpoB). We show that rifampicin exposure results in rpoB upregulation in a sub-population of cells, followed by growth. More specifically, rifampicin preferentially inhibits one of the two rpoB promoters (promoter I), allowing increased rpoB expression from a second promoter (promoter II), and thus triggering growth. Disruption of promoter architecture leads to differences in rifampicin susceptibility of the population, confirming the contribution of rifampicin-induced rpoB expression to tolerance.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Aldridge, B. B., Keren, I. & Fortune, S. M. The spectrum of drug susceptibility in Mycobacteria. Microbiol. Spectrum.10.1128/microbiolspec.MGM2-0031-2013 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

