Non-neutralizing antibodies elicited by recombinant Lassa-Rabies vaccine are critical for protection against Lassa fever
- PMID: 30310067
- PMCID: PMC6181965
- DOI: 10.1038/s41467-018-06741-w
Non-neutralizing antibodies elicited by recombinant Lassa-Rabies vaccine are critical for protection against Lassa fever
Abstract
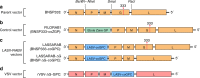
Lassa fever (LF), caused by Lassa virus (LASV), is a viral hemorrhagic fever for which no approved vaccine or potent antiviral treatment is available. LF is a WHO priority disease and, together with rabies, a major health burden in West Africa. Here we present the development and characterization of an inactivated recombinant LASV and rabies vaccine candidate (LASSARAB) that expresses a codon-optimized LASV glycoprotein (coGPC) and is adjuvanted by a TLR-4 agonist (GLA-SE). LASSARAB elicits lasting humoral response against LASV and RABV in both mouse and guinea pig models, and it protects both guinea pigs and mice against LF. We also demonstrate a previously unexplored role for non-neutralizing LASV GPC-specific antibodies as a major mechanism of protection by LASSARAB against LF through antibody-dependent cellular functions. Overall, these findings demonstrate an effective inactivated LF vaccine and elucidate a novel humoral correlate of protection for LF.
Conflict of interest statement
T.A.-M, P.B.J., and M.J.S. are inventors on the U.S. Provisional Patent Application No. 62/691,413 (Title: Non-neutralizing antibodies elicited by recombinant Lassa–Rabies vaccine are critical for protection against Lassa fever). All remaining authors declare no competing interests.
Figures




References
-
- Nigerian Centre for Disease Control. An update of Lassa fever outbreak in Nigeria, Vol. 19, May Report. (ed. Nigeria Centre for Disease Control) 1–6 (2018).
Publication types
MeSH terms
Substances
Grants and funding
- HHSN272200700016I/AO/NIAID NIH HHS/United States
- R01 AI105204/AI/NIAID NIH HHS/United States
- Intramural/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- 5R01AI105204-05/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

