Characterizing the protective effects of SHLP2, a mitochondrial-derived peptide, in macular degeneration
- PMID: 30310092
- PMCID: PMC6182005
- DOI: 10.1038/s41598-018-33290-5
Characterizing the protective effects of SHLP2, a mitochondrial-derived peptide, in macular degeneration
Abstract
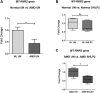
Mitochondrial-derived peptides (MDPs) are rapidly emerging therapeutic targets to combat development of neurodegenerative diseases. SHLP2 (small humanin-like peptide 2) is a newly discovered MDP that is coded from the MT-RNR2 (Mitochondrially encoded 16S rRNA) gene in mitochondrial DNA (mtDNA). In the current study, we examined the biological consequences of treatment with exogenously-added SHLP2 in an in vitro human transmitochondrial age-related macular degeneration (AMD) ARPE-19 cell model. In AMD cells, we observed significant down-regulation of the MDP-coding MT-RNR2 gene, and remarkably reduced levels of all five oxidative phosphorylation (OXPHOS) complex I-V protein subunits that are involved in the electron transport chain; these results suggested mitochondrial toxicity and abnormal OXPHOS complex protein subunits' levels in AMD cells. However, treatment of AMD cells with SHLP2: (1) restored the normal levels of OXPHOS complex protein subunits, (2) prevented loss of viable cells and mitochondria, (3) increased the number of mtDNA copies, (4) induced anti-apoptotic effects, and (5) attenuated amyloid-β-induced cellular and mitochondrial toxicity. Cumulatively, our findings established the protective role of SHLP2 in AMD cells in vitro. In conclusion, this novel study supports the merit of SHLP2 in the treatment of AMD, a primary retinal disease that is a leading cause of blindness among the elderly population in the United States as well as worldwide.
Conflict of interest statement
S.N. None; P.C. Consultant and stockholder of CohBar Inc. A.B.N. None; B.D.K.
Figures