PKCβII-induced upregulation of PGP9.5 and VEGF in postoperative persistent pain in rats
- PMID: 30310311
- PMCID: PMC6166760
- DOI: 10.2147/JPR.S144852
PKCβII-induced upregulation of PGP9.5 and VEGF in postoperative persistent pain in rats
Abstract
Purpose: Postoperative pain is a common clinical problem. In this study, we aimed to investigate the role of protein kinase C βII (PKCβII) in the progression of postoperative pain following skin/muscle incision and retraction (SMIR) surgery.
Materials and methods: SMIR postoperative pain model was established in rats, akin to a clinical procedure. The expression level and location of p-PKCβII were observed in dorsal root ganglion (DRG) or spinal cord from SMIR-operated rats by Western blotting and immunofluorescence. In addition, the effects of PKCβII on the expression of protein gene product 9.5 (PGP9.5) or vascular endothelial growth factor (VEGF) were assessed by using pharmacological activator and inhibitor of PKCβII. Moreover, mechanical withdrawal threshold (MWT) was assessed before or after SMIR-operated rats were treated with inhibitor or activator of PKCβII.
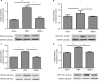
Results: The expression of PKCβII in DRG and spinal cord was significantly increased after SMIR surgery (P < 0.001, P < 0.01) and expression of PKCβII was located in the neurons of the spinal cord, and magnocellular neurons, non-peptide neurons, and peptide neurons in DRG. Besides, compared with skin/muscle incision group, retraction caused a marked increase in the expression of PKCβII and a significant decrease of MWT (P < 0.001, P < 0.05). The activator of PKCβII greatly increased the expression of PGP9.5 and VEGF (P < 0.05, P < 0.01) and enhanced MWT (P < 0.001), while inhibitor of PKCβII decreased the expression of PGP9.5 and VEGF and attenuated MWT (P < 0.05, P < 0.01, P < 0.001).
Conclusion: Activation of PKCβII signaling pathways might be an important mechanism in the progression of postoperative pain.
Keywords: PGP9.5; PKCβII; VEGF; neurons; postoperative persistent pain.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures









Similar articles
-
Skin/muscle incision and retraction regulates the persistent postoperative pain in rats by the Epac1/PKC-βII pathway.BMC Anesthesiol. 2022 Jul 18;22(1):230. doi: 10.1186/s12871-022-01771-w. BMC Anesthesiol. 2022. PMID: 35850627 Free PMC article.
-
Effect of Epac1 on pERK and VEGF Activation in Postoperative Persistent Pain in Rats.J Mol Neurosci. 2016 Aug;59(4):554-64. doi: 10.1007/s12031-016-0776-x. Epub 2016 Jun 10. J Mol Neurosci. 2016. PMID: 27287221
-
p38 and interleukin-1 beta pathway via toll-like receptor 4 contributed to the skin and muscle incision and retraction-induced allodynia.J Surg Res. 2015 Aug;197(2):339-47. doi: 10.1016/j.jss.2015.04.061. Epub 2015 Apr 21. J Surg Res. 2015. PMID: 25979559
-
Forced treadmill running suppresses postincisional pain and inhibits upregulation of substance P and cytokines in rat dorsal root ganglion.J Pain. 2014 Aug;15(8):827-34. doi: 10.1016/j.jpain.2014.04.010. Epub 2014 May 20. J Pain. 2014. PMID: 24854064
-
High-frequency transcutaneous electrical nerve stimulation attenuates postsurgical pain and inhibits excess substance P in rat dorsal root ganglion.Reg Anesth Pain Med. 2014 Jul-Aug;39(4):322-8. doi: 10.1097/AAP.0000000000000091. Reg Anesth Pain Med. 2014. PMID: 24781287
Cited by
-
Skin/muscle incision and retraction regulates the persistent postoperative pain in rats by the Epac1/PKC-βII pathway.BMC Anesthesiol. 2022 Jul 18;22(1):230. doi: 10.1186/s12871-022-01771-w. BMC Anesthesiol. 2022. PMID: 35850627 Free PMC article.
-
Regulation of CaV3.2 channels by the receptor for activated C kinase 1 (Rack-1).Pflugers Arch. 2022 Apr;474(4):447-454. doi: 10.1007/s00424-021-02631-1. Epub 2021 Oct 8. Pflugers Arch. 2022. PMID: 34623515
-
Protective Roles of Apigenin Against Cardiometabolic Diseases: A Systematic Review.Front Nutr. 2022 Apr 15;9:875826. doi: 10.3389/fnut.2022.875826. eCollection 2022. Front Nutr. 2022. PMID: 35495935 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

