Near-infrared fluorescence probes to detect reactive oxygen species for keloid diagnosis
- PMID: 30310562
- PMCID: PMC6115726
- DOI: 10.1039/c8sc01865k
Near-infrared fluorescence probes to detect reactive oxygen species for keloid diagnosis
Abstract
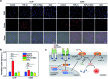
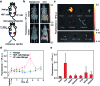
Development of molecular probes for the detection of reactive oxygen and nitrogen species (RONS) is important for the pathology and diagnosis of diseases. Although an abnormally high RONS level has been identified in keloids - a benign dermal tumour developed after lesion, the ability of employing RONS probes for keloid detection has not yet been exploited. Herein, we report two near-infrared (NIR) fluorescent probes (CyTF and CyBA) that can specifically distinguish keloid fibroblasts from normal dermal fibroblasts. Both CyTF and CyBA show a 15-fold NIR fluorescence enhancement at 717 nm upon reaction with RONS. However, because CyTF has higher specificity towards ONOO- than CyBA, CyTF can detect stimulated fibroblasts in a more sensitive way, showing 3.76 and 2.26-fold fluorescence increments in TGF-β1 stimulated dermal fibroblasts and keloid fibroblasts, respectively. Furthermore, CyTF permits specific detection of implanted keloid fibroblasts in a xenograft live mouse model. Our work thus developed a new optical imaging approach that has the potential for early diagnosis and drug screening of keloids.
Figures




References
-
- Medzhitov R. Nature. 2008;454:428–435. - PubMed
-
- De Felice B., Garbi C., Santoriello M., Santillo A., Wilson R. R. Mol. Cell. Biochem. 2009;327:191–201. - PubMed
-
- Wolfram D., Tzankov A., Pulzl P., Piza-Katzer H. Dermatol. Surg. 2009;35:171–181. - PubMed
-
- Miao Q., Yeo D. C., Wiraja C., Zhang J., Ning X., Xu C., Pu K. Angew. Chem., Int. Ed. Engl. 2018;57:1256–1260. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

