Sensitized triplet-triplet annihilation upconversion in water and its application to photochemical transformations
- PMID: 30310600
- PMCID: PMC6115628
- DOI: 10.1039/c8sc01829d
Sensitized triplet-triplet annihilation upconversion in water and its application to photochemical transformations
Abstract
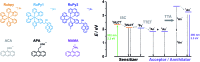
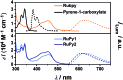
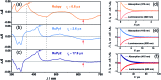
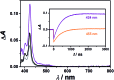
Sensitized triplet-triplet annihilation (TTA) is a promising mechanism for solar energy conversion, but so far its application has been practically completely limited to organic solvents and self-assembled or solid state systems. Combining water-soluble ruthenium complex-pyrene dyads with particularly long excited-state lifetimes as sensitizers and highly fluorescent commercial anthracenes as acceptors/annihilators, we were able to achieve green-to-violet upconversion with unprecedented quantum yields in pure water. Compared to the only known system exploiting sensitized TTA in homogeneous aqueous solution, we improve the overall photon upconversion efficiency by a full order of magnitude and present the very first example for a chemical transformation on a laboratory scale via upconversion in water. Specifically, we found that a thermodynamically challenging carbon-chlorine bond activation can be driven by green photons from an inexpensive continuous wave light source in the presence of dissolved oxygen. Our study is thus potentially relevant in the context of cleaning water from halogenated (toxic) contaminants and for sustainable photochemistry in the most environmentally friendly solvent.
Figures



References
-
- Singh-Rachford T. N., Castellano F. N. Coord. Chem. Rev. 2010;254:2560–2573.
-
- Zhao J., Ji S., Guo H. RSC Adv. 2011;1:937–950.
-
- Gray V., Dzebo D., Abrahamsson M., Albinsson B., Moth-Poulsen K. Phys. Chem. Chem. Phys. 2014;16:10345–10352. - PubMed
-
- Guo X., Liu Y., Chen Q., Zhao D., Ma Y. Adv. Opt. Mater. 2018;6:1700981.
-
- Zhou J., Liu Q., Feng W., Sun Y., Li F. Chem. Rev. 2015;115:395–465. - PubMed
LinkOut - more resources
Full Text Sources

