Programmable one-pot synthesis of heparin pentasaccharides enabling access to regiodefined sulfate derivatives
- PMID: 30310602
- PMCID: PMC6115620
- DOI: 10.1039/c8sc01743c
Programmable one-pot synthesis of heparin pentasaccharides enabling access to regiodefined sulfate derivatives
Abstract
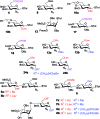
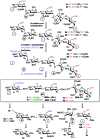
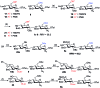
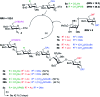
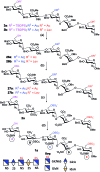
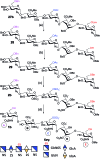
Heparin (H) and heparan sulfate (HS) belong to the glycosaminoglycan (GAG) family of oligosaccharides, and their sequences and sulfation patterns are known to regulate the functions of various proteins in biological processes. Among these, the 6-O-sulfation of HS/H contributes most significantly to the structural diversity and binding interactions. However, the synthesis of HS with defined sulfation patterns remains a major challenge. Herein, we report a highly efficient and programmable one-pot method for the synthesis of protected heparin pentasaccharides using thioglycoside building blocks with optimized relative reactivities to allow the selective deprotection and preparation of regiodefined sulfate derivatives.
Figures
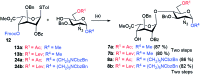
References
-
- Poulain F. E., Yost H. J., Fu L., Suflita M., Linhardt R. J., Capila I., Linhardt R. J. Development. Adv. Drug Delivery Rev. Angew. Chem. 2015;2016;2002;14297114:3456. 237, 426. - PubMed
-
- Xu D., Esko J. Annu. Rev. Biochem. 2014;83:129. - PMC - PubMed
- Linhardt R. J. J. Med. Chem. 2003;46:2551. - PubMed
- Meneghetti M. C. Z., Hughes A. J., Rudd T. R., Nader H. B., Powell A. K., Yates E. A., Lima M. A. J. R. Soc., Interface. 2015;12:20150589. - PMC - PubMed
- Parra A., Veraldi N., Locatelli M., Fini M., Martini L., Torri G., Sangiorgi L., Bisio A. Glycobiology. 2012;22:248. - PubMed
-
- Linhardt R. J. and Toida T., in Carbohydrates in Drug design, ed. Z. B. Witczak and K. A. Nieforth, Marcel Dekker, New York, 1997, ch. 4, p. 277.
Grants and funding
LinkOut - more resources
Full Text Sources

