Nickel(0)-catalyzed linear-selective hydroarylation of unactivated alkenes and styrenes with aryl boronic acids
- PMID: 30310616
- PMCID: PMC6115005
- DOI: 10.1039/c8sc02101e
Nickel(0)-catalyzed linear-selective hydroarylation of unactivated alkenes and styrenes with aryl boronic acids
Abstract
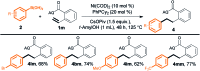
Herein, we describe the first linear-selective hydroarylation reaction of unactivated alkenes and styrenes with aryl boronic acids, which was achieved by introducing a directing group on the alkenes. This efficient, scalable reaction serves as a method for modular assembly of structurally diverse alkyl arenes, including γ-aryl butyric acid derivatives, which are widely utilized as chemical building blocks for the synthesis of various drugs and other biologically active compounds.
Figures





References
-
-
For some reviews on metal-hydride chemistry, see:
- Rendler S., Oestreich M. Angew. Chem., Int. Ed. 2007;46:498. - PubMed
- Deutsch C., Krause N., Lipshutz B. H. Chem. Rev. 2008;108:2916. - PubMed
- Pirnot M. T., Wang Y.-M., Buchwald S. L. Angew. Chem., Int. Ed. 2016;55:48. - PMC - PubMed
- Greenhalgh M. D., Jones A. S., Thomas S. P. ChemCatChem. 2015;7:190.
- Maksymowicz R. M., Bissette A. J., Fletcher S. P. Chem.–Eur. J. 2015;21:5668. - PubMed
- Crossley S. W. M., Obradors C., Martinez R. M., Shenvi R. A. Chem. Rev. 2016;116:8912. - PMC - PubMed
- Nguyen K. D., Park B. Y., Luong T., Sato H., Garza V. J., Krische M. J. Science. 2016;354:300. - PMC - PubMed
-
-
-
For some reviews on hydroarylation of alkenes, see:
- Kakiuchi F., Murai S. Acc. Chem. Res. 2002;35:826. - PubMed
- Pan S., Shibata T. ACS Catal. 2013;3:704.
- Zeng X. Chem. Rev. 2013;113:6864. - PubMed
- Yang L., Huang H. Chem. Rev. 2015;115:3468. - PubMed
- Crisenza G. E. M., Bower J. F. Chem. Lett. 2016;45:2.
- Dong Z., Ren Z., Thompson S. J., Xu Y., Dong G. Chem. Rev. 2017;117:9333. - PubMed
-
-
-
For some examples on hydroarylation of alkenes using arenes as the aryl source, see:
- Sun Z.-M., Zhang J., Manan R. S., Zhao P. J. Am. Chem. Soc. 2010;132:6935. - PubMed
- Nakao Y., Yamada Y., Kashihara N., Hiyama T. J. Am. Chem. Soc. 2010;132:13666. - PubMed
- Gao K., Yoshikai N. J. Am. Chem. Soc. 2011;133:400. - PubMed
- Guan B.-T., Hou Z. J. Am. Chem. Soc. 2011;133:18086. - PubMed
- Oyamada J., Hou Z. Angew. Chem., Int. Ed. 2012;51:12828. - PubMed
- Pan S., Ryu N., Shibata T. J. Am. Chem. Soc. 2012;134:17474. - PubMed
- Lee P.-S., Yoshikai N. Angew. Chem., Int. Ed. 2013;52:1240. - PubMed
- Andou T., Saga Y., Komai H., Matsunaga S., Kanai M. Angew. Chem., Int. Ed. 2013;52:3213. - PubMed
- Xu W., Yoshikai N. Angew. Chem., Int. Ed. 2014;53:14166. - PubMed
- Crisenza G. E. M., McCreanor N. G., Bower J. F. J. Am. Chem. Soc. 2014;136:10258. - PubMed
- Bair J. S., Schramm Y., Sergeev A. G., Clot E., Eisenstein O., Hartwig J. F. J. Am. Chem. Soc. 2014;136:13098. - PubMed
- Crisenza G. E. M., Sokolova O. O., Bower J. F. Angew. Chem., Int. Ed. 2015;54:14866. - PMC - PubMed
- Schramm Y., Takeuchi M., Semba K., Nakao Y., Hartwig J. F. J. Am. Chem. Soc. 2015;137:12215. - PubMed
- Ebe Y., Nishimura T. J. Am. Chem. Soc. 2015;137:5899. - PubMed
- Filloux C. M., Rovis T. J. Am. Chem. Soc. 2015;137:508. - PMC - PubMed
- Hatano M., Ebe Y., Nishimura T., Yorimitsu H. J. Am. Chem. Soc. 2016;138:4010. - PubMed
- Kimura N., Kochi T., Kakiuchi F. J. Am. Chem. Soc. 2017;139:14849. - PubMed
- Loup J., Zell D., Oliveira J. C. A., Keil H., Stalke D., Ackermann L. Angew. Chem., Int. Ed. 2017;56:14197. - PubMed
-
-
- Semba K., Ariyama K., Zheng H., Kameyama R., Sakaki S., Nakao Y. Angew. Chem., Int. Ed. 2016;55:6275. - PubMed
- Friis S. D., Pirnot M. T., Buchwald S. L. J. Am. Chem. Soc. 2016;138:8372. - PMC - PubMed
- Friis S. D., Pirnot M. T., Dupuis L. N., Buchwald S. L. Angew. Chem., Int. Ed. 2017;56:7242. - PMC - PubMed
- Lu X., Xiao B., Zhang Z., Gong T., Su W., Yi J., Fu Y., Liu L. Nat. Commun. 2016;7:11129. - PMC - PubMed
- Green S. A., Matos J. L. M., Yagi A., Shenvi R. A. J. Am. Chem. Soc. 2016;138:12779. - PubMed
- He Y., Cai Y., Zhu S. J. Am. Chem. Soc. 2017;139:1061. - PubMed
- Chen F., Chen K., Zhang Y., He Y., Wang Y.-M., Zhu S. J. Am. Chem. Soc. 2017;139:13929. - PubMed
- Li W., Boon J. K., Zhao Y. Chem. Sci. 2018;9:600. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

