Less toxic zinc(ii), diorganotin(iv), gallium(iii) and cadmium(ii) complexes derived from 2-benzoylpyridine N, N-dimethylthiosemicarbazone: synthesis, crystal structures, cytotoxicity and investigations of mechanisms of action
- PMID: 30310676
- PMCID: PMC6116716
- DOI: 10.1039/c8tx00127h
Less toxic zinc(ii), diorganotin(iv), gallium(iii) and cadmium(ii) complexes derived from 2-benzoylpyridine N, N-dimethylthiosemicarbazone: synthesis, crystal structures, cytotoxicity and investigations of mechanisms of action
Abstract
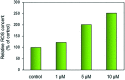
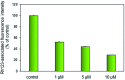
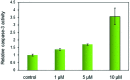
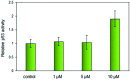
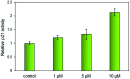
Four metal complexes based on 2-benzoylpyridine N,N-dimethylthiosemicarbazone (Bp44mT) were designed. Free ligand and zinc(ii), diorganotin(iv), gallium(iii) and cadmium(ii) complexes all demonstrated pronounced activity, which was indicated using the growth inhibition test in vitro. Interestingly, most of the compounds were found to be selective against hepatocellular carcinoma (HepG2) cells but had little effect on normal hepatocyte (QSG7701) cells. In particular, Zn(Bp44mT)2 (1) exhibited toxicity on QSG7701 cells which approximately 12-fold lower than that on HepG2 cells. The studies of mechanisms of action indicated that 1 induced reactive oxygen species (ROS) generation in a dose-dependent manner via the mitochondria transduction pathway. Protein analyses showed that 1 significantly promoted p21 and p53 gene expression, causing caspase-3 activation.
Figures


References
-
- Motswainyana W. M., Onani M. O., Madiehe A. M. Polyhedron. 2012;41:44–51.
-
- Ramachandran E., Raja D. S., Rath N. P., Natarajan K. Inorg. Chem. 2013;52:1504–1514. - PubMed
-
- Chakraborty A., Dash S. P., Panda A. K., Acharyya R., Biswas A., Mukhopadhyay S., Dinda R. Dalton Trans. 2015;44:6140–6157. - PubMed
-
- Raja D. S., Bhuvanesh N. S., Natarajan K. Dalton Trans. 2012;41:4365–4377. - PubMed
-
- Islam A., Rodrigues B. L., Marzano I. M., Perreira-Maia E. C., Dittz D., Lopes M. T. P., Demicheli C. Eur. J. Med. Chem. 2016;109:254–267. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

