ClinVar database of global familial hypercholesterolemia-associated DNA variants
- PMID: 30311388
- PMCID: PMC6206854
- DOI: 10.1002/humu.23634
ClinVar database of global familial hypercholesterolemia-associated DNA variants
Abstract
Accurate and consistent variant classification is imperative for incorporation of rapidly developing sequencing technologies into genomic medicine for improved patient care. An essential requirement for achieving standardized and reliable variant interpretation is data sharing, facilitated by a centralized open-source database. Familial hypercholesterolemia (FH) is an exemplar of the utility of such a resource: it has a high incidence, a favorable prognosis with early intervention and treatment, and cascade screening can be offered to families if a causative variant is identified. ClinVar, an NCBI-funded resource, has become the primary repository for clinically relevant variants in Mendelian disease, including FH. Here, we present the concerted efforts made by the Clinical Genome Resource, through the FH Variant Curation Expert Panel and global FH community, to increase submission of FH-associated variants into ClinVar. Variant-level data was categorized by submitter, variant characteristics, classification method, and available supporting data. To further reform interpretation of FH-associated variants, areas for improvement in variant submissions were identified; these include a need for more detailed submissions and submission of supporting variant-level data, both retrospectively and prospectively. Collaborating to provide thorough, reliable evidence-based variant interpretation will ultimately improve the care of FH patients.
Keywords: ClinVar; Clinical Genome Resource; familial hypercholesterolemia; variant interpretation.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures
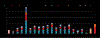
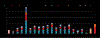
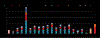




References
-
- Alves AC, Etxebarria A, Soutar AK, Martin C, & Bourbon M (2014). Novel functional APOB mutations outside LDL-binding region causing familial hypercholesterolaemia. Human Molecular Genetics, 23, 1817–1828. - PubMed
-
- Bland A, Harrington EA, Dunn K, Pariani M, Platt JCK, Grove M, & Caleshu C (2018). Clinically impactful difference in variant interpretation between clinicians and tetsing laboraoties: a single-center experience. Genetics in Medicine, 20, 369–373. - PubMed
-
- Blueprint Genetics. (2016). A guide to understanding variant classification Retrieved April 11, 2018, from https://submit.ncbi.nlm.nih.gov/ft/byid/nxpnxkpc/variant_classification_...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

