Genome amplification and cellular senescence are hallmarks of human placenta development
- PMID: 30312291
- PMCID: PMC6200260
- DOI: 10.1371/journal.pgen.1007698
Genome amplification and cellular senescence are hallmarks of human placenta development
Abstract
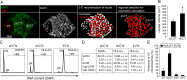
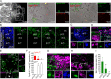
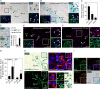
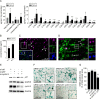
Genome amplification and cellular senescence are commonly associated with pathological processes. While physiological roles for polyploidization and senescence have been described in mouse development, controversy exists over their significance in humans. Here, we describe tetraploidization and senescence as phenomena of normal human placenta development. During pregnancy, placental extravillous trophoblasts (EVTs) invade the pregnant endometrium, termed decidua, to establish an adapted microenvironment required for the developing embryo. This process is critically dependent on continuous cell proliferation and differentiation, which is thought to follow the classical model of cell cycle arrest prior to terminal differentiation. Strikingly, flow cytometry and DNAseq revealed that EVT formation is accompanied with a genome-wide polyploidization, independent of mitotic cycles. DNA replication in these cells was analysed by a fluorescent cell-cycle indicator reporter system, cell cycle marker expression and EdU incorporation. Upon invasion into the decidua, EVTs widely lose their replicative potential and enter a senescent state characterized by high senescence-associated (SA) β-galactosidase activity, induction of a SA secretory phenotype as well as typical metabolic alterations. Furthermore, we show that the shift from endocycle-dependent genome amplification to growth arrest is disturbed in androgenic complete hydatidiform moles (CHM), a hyperplastic pregnancy disorder associated with increased risk of developing choriocarinoma. Senescence is decreased in CHM-EVTs, accompanied by exacerbated endoreduplication and hyperploidy. We propose induction of cellular senescence as a ploidy-limiting mechanism during normal human placentation and unravel a link between excessive polyploidization and reduced senescence in CHM.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Genome multiplication of extravillous trophoblast cells in human placenta in the course of differentiation and invasion into endometrium and myometrium. II. Mechanisms of polyploidization.Tsitologiia. 2004;46(7):640-8. Tsitologiia. 2004. PMID: 15473375
-
Genome multiplication of extravillous trophoblast cells in human placenta in the course of differentiation and invasion into endometrium and myometrium. I. Dynamics of polyploidization.Tsitologiia. 2002;44(11):1058-67. Tsitologiia. 2002. PMID: 12561726
-
Genome multiplication in the tertiary giant trophoblast cells in the course of their endovascular and interstitial invasion into the rat placenta decidua basalis.Early Pregnancy (Cherry Hill). 2000 Apr;4(2):99-109. Early Pregnancy (Cherry Hill). 2000. PMID: 11723540
-
Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Environment.Front Immunol. 2018 Nov 13;9:2597. doi: 10.3389/fimmu.2018.02597. eCollection 2018. Front Immunol. 2018. PMID: 30483261 Free PMC article. Review.
-
Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction?Am J Obstet Gynecol. 2018 Feb;218(2S):S762-S773. doi: 10.1016/j.ajog.2017.11.567. Epub 2017 Dec 22. Am J Obstet Gynecol. 2018. PMID: 29275823 Review.
Cited by
-
Feto-placental Unit: From Development to Function.Adv Exp Med Biol. 2023;1428:1-29. doi: 10.1007/978-3-031-32554-0_1. Adv Exp Med Biol. 2023. PMID: 37466767 Review.
-
Abnormal Cullin1 neddylation-mediated p21 accumulation participates in the pathogenesis of recurrent spontaneous abortion by regulating trophoblast cell proliferation and differentiation.Mol Hum Reprod. 2020 May 15;26(5):327-339. doi: 10.1093/molehr/gaaa021. Mol Hum Reprod. 2020. PMID: 32186736 Free PMC article.
-
Regulation of p27Kip1 and p57Kip2 Functions by Natural Polyphenols.Biomolecules. 2020 Sep 13;10(9):1316. doi: 10.3390/biom10091316. Biomolecules. 2020. PMID: 32933137 Free PMC article. Review.
-
Dysregulation of Placental Functions and Immune Pathways in Complete Hydatidiform Moles.Int J Mol Sci. 2019 Oct 10;20(20):4999. doi: 10.3390/ijms20204999. Int J Mol Sci. 2019. PMID: 31658584 Free PMC article.
-
Utilizing primary HLA-G+ extravillous trophoblasts and HLA-G+ EVT-like cell lines to study maternal-fetal interactions.STAR Protoc. 2023 May 11;4(2):102276. doi: 10.1016/j.xpro.2023.102276. Online ahead of print. STAR Protoc. 2023. PMID: 37178111 Free PMC article.
References
-
- Hamilton WJ, Boyd JD. Trophoblast in human utero-placental arteries. Nature. 1966;212(5065):906–8. . - PubMed
-
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1(1):3–19. . - PubMed
-
- Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, et al. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(10):4012–7. 10.1073/pnas.1005342108 ; PubMed Central PMCID: PMC3053985. - DOI - PMC - PubMed
-
- Tilburgs T, Evans JH, Crespo AC, Strominger JL. The HLA-G cycle provides for both NK tolerance and immunity at the maternal-fetal interface. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(43):13312–7. 10.1073/pnas.1517724112 ; PubMed Central PMCID: PMC4629323. - DOI - PMC - PubMed

