Streptomycetes: Surrogate hosts for the genetic manipulation of biosynthetic gene clusters and production of natural products
- PMID: 30312648
- PMCID: PMC6343487
- DOI: 10.1016/j.biotechadv.2018.10.003
Streptomycetes: Surrogate hosts for the genetic manipulation of biosynthetic gene clusters and production of natural products
Abstract
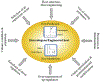
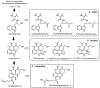
Due to the worldwide prevalence of multidrug-resistant pathogens and high incidence of diseases such as cancer, there is an urgent need for the discovery and development of new drugs. Nearly half of the FDA-approved drugs are derived from natural products that are produced by living organisms, mainly bacteria, fungi, and plants. Commercial development is often limited by the low yield of the desired compounds expressed by the native producers. In addition, recent advances in whole genome sequencing and bioinformatics have revealed an abundance of cryptic biosynthetic gene clusters within microbial genomes. Genetic manipulation of clusters in the native host is commonly used to awaken poorly expressed or silent gene clusters, however, the lack of feasible genetic manipulation systems in many strains often hinders our ability to engineer the native producers. The transfer of gene clusters into heterologous hosts for expression of partial or entire biosynthetic pathways is an approach that can be used to overcome this limitation. Heterologous expression also facilitates the chimeric fusion of different biosynthetic pathways, leading to the generation of "unnatural" natural products. The genus Streptomyces is especially known to be a prolific source of drugs/antibiotics, its members are often used as heterologous expression hosts. In this review, we summarize recent applications of Streptomyces species, S. coelicolor, S. lividans, S. albus, S. venezuelae and S. avermitilis, as heterologous expression systems.
Keywords: Biosynthetic gene clusters; Combinatorial biosynthesis; Heterologous expression; Natural products; Streptomyces.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


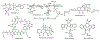


References
-
- Alt S, and Wilkinson B, 2015. Biosynthesis of the novel macrolide antibiotic anthracimycin. ACS Chem. Biol. 10(11), 2468–2479. - PubMed
-
- Awakawa T, Zhang L, Wakimoto T, Hoshino S, Mori T, Ito T, Ishikawa J, Tanner ME, and Abe I, 2014. A methyltransferase initiates terpene cyclization in teleocidin B biosynthesis. J. Am. Chem. Soc, 136(28), 9910–9913. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

