Risk of regorafenib-induced cardiovascular events in patients with solid tumors: A systematic review and meta-analysis
- PMID: 30313066
- PMCID: PMC6203579
- DOI: 10.1097/MD.0000000000012705
Risk of regorafenib-induced cardiovascular events in patients with solid tumors: A systematic review and meta-analysis
Abstract
Background: The present comparative meta-analysis was conducted to evaluate the cardiovascular events of regorafenib in patients with solid tumors.
Methods: Eligible studies from MEDLINE, Google Scholar, Cochrane Library, Clinical key, EBSCO publishing and Ovid, which had reported cardiovascular adverse events potentially caused by regorafenib were absorbed. Data of clinical characteristics and cardiovascular events including hypertension, hemorrhage, thrombosis, and heart failure were extracted from selected literatures for the final analysis. Pooled analysis of cardiovascular adverse events was developed by relative risks (RRs) and corresponding 95% confidence intervals (CIs) with software STATA 13.0 and RevMan 5.3.
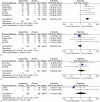
Results: Thirty studies including 3813 patients were fit into analysis. The incidences of cardiovascular events of all-grade were: hypertension, 36.8% (95% CI, 29.8%-43.8%), hemorrhage, 8.6% (95% CI, 3.2%-14%), thrombosis, 1.4% (95% CI, 0.1%-2.8%), and heart failure, 2.9% (95% CI, 0.3%-5.6%). The incidences of cardiovascular events of high-grade were: hypertension, 9.9% (95% CI, 7.4%-12.4%), hemorrhage, 1.2% (95% CI, 0.3%-2.2%), thrombosis, 1.6% (95% CI, 0.2%-3.4%), and heart failure, 2.9% (95% CI, 0.3%-5.6%). The RRs and their 95% CIs of all-grade cardiovascular events among patients treated with regorafenib were: hypertension, 4.10 (95% CI, 3.07-5.46; P < .00001), hemorrhage, 2.71 (95% CI, 1.45-5.08; P = .002), thrombosis, 1.27 (95% CI, 0.49-3.27; P = .62), and heart failure, 0.79 (95% CI, 0.16-3.94; P = .77). The RRs and their 95% CIs of high-grade cardiovascular events among patients treated with regorafenib were: hypertension, 5.82 (95% CI, 3.46-9.78; P < .00001), hemorrhage, 0.90 (95% CI, 0.50-1.61; P = .72), thrombosis, 1.28 (95% CI, 0.48-3.41; P = .62), and heart failure, 1.15 (95% CI, 0.23-5.69; P = .86), respectively.
Conclusion: The present meta-analysis has demonstrated that regorafenib is associated with an increasing risk of hypertension at all-grade and high-grade, as well as hemorrhage at all-grade. Adequate awareness of cardiovascular adverse events of regorafenib should be established for clinicians.
Conflict of interest statement
Authors declare that they have no conflicts of interest.
Figures




References
-
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–55. - PubMed
-
- Strumberg D, Schultheis B. Regorafenib for cancer. Exp Opin Investig Drugs 2012;21:879–89. - PubMed
-
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. - PubMed
-
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

