Molecular Imaging of the Cholinergic System in Parkinson's Disease
- PMID: 30314597
- PMCID: PMC6218162
- DOI: 10.1016/bs.irn.2018.07.027
Molecular Imaging of the Cholinergic System in Parkinson's Disease
Abstract
One of the first identified neurotransmitters in the brain, acetylcholine, is an important modulator that drives changes in neuronal and glial activity. For more than two decades, the main focus of molecular imaging of the cholinergic system in Parkinson's disease (PD) has been on cognitive changes. Imaging studies have confirmed that degeneration of the cholinergic system is a major determinant of dementia in PD. Within the last decade, the focus is expanding to studying cholinergic correlates of mobility impairments, dyskinesias, olfaction, sleep, visual hallucinations and risk taking behavior in this disorder. These studies increasingly recognize that the regional topography of cholinergic brain areas associates with specific functions. In parallel with this trend, more recent molecular cholinergic imaging approaches are investigating cholinergic modulatory functions and contributions to large-scale brain network functions. A novel area of research is imaging cholinergic innervation functions of peripheral autonomic organs that may have the potential of future prodromal diagnosis of PD. Finally, emerging evidence of hypercholinergic activity in prodromal and symptomatic leucine-rich repeat kinase 2 PD may reflect neuronal cholinergic compensation versus a response to neuro-inflammation. Molecular imaging of the cholinergic system has led to many new insights in the etiology of dopamine non-responsive symptoms of PD (more "malignant" hypocholinergic disease phenotype) and is poised to guide and evaluate future cholinergic drug development in this disorder.
Keywords: Acetylcholine; Brain network; Cognition; Dementia with Lewy bodies; Gait; Malignant phenotype; Motor; Parkinson's disease.
© 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing interests: The authors report no competing interests.
Figures
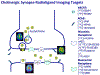
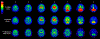

References
-
- Ahlskog JE, & Muenter MD (2001). Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord, 16(3), 448–458. - PubMed
-
- Alves G, Larsen JP, Emre M, Wentzel-Larsen T, & Aarsland D (2006). Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord, 21(8), 1123–1130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

