Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease
- PMID: 30314894
- PMCID: PMC6286638
- DOI: 10.1016/j.ebiom.2018.10.006
Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease
Erratum in
-
Corrigendum to "Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease" [EBioMedicine 37 (2018) 269-280].EBioMedicine. 2021 Jan;63:103073. doi: 10.1016/j.ebiom.2020.103073. Epub 2021 Jan 6. EBioMedicine. 2021. PMID: 33418510 Free PMC article. No abstract available.
Abstract
Background: Acute kidney injury (AKI) may lead to the development of chronic kidney disease (CKD), i.e. AKI-CKD transition, but the underlying mechanism remains largely unclear. Endoplasmic reticulum (ER) stress is characterized by the accumulation of unfolded or misfolded proteins in ER resulting in a cellular stress response. The role of ER stress in AKI-CKD transition remains unknown.
Methods: In this study, we examined ER stress in the mouse model of AKI-CKD transition after unilateral renal ischemia-reperfusion injury (uIR). To determine the role of ER stress in AKI-CKD transition, we tested the effects of two chemical chaperones: Tauroursodeoxycholic acid (TUDCA) and 4-phenylbutyric acid (4-PBA).
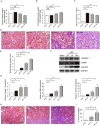
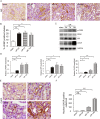
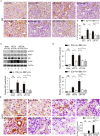
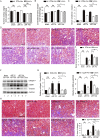
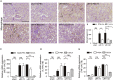
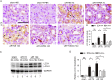
Findings: uIR led to the induction of ER stress in kidneys, as indicated by increased expression of UPR molecules CHOP (C/EBP homologous protein) and BiP(binding immunoglobulin protein; also called GRP78-78 kDa glucose-regulated protein). Given at 3 days after uIR, both TUDCA and 4-PBA blocked ER stress in post-ischemic kidneys. Notably, both chemicals promoted renal recovery and suppressed tubulointerstitial injury as manifested by the reduction of tubular atrophy, renal fibrosis and myofibroblast activation. Inhibition of ER stress further attenuated renal tubular epithelial cell apoptosis, inflammation and autophagy in post-ischemic kidneys.
Interpretation: These findings suggest that ER stress contributes critically to the development of chronic kidney pathologies and CKD following AKI, and inhibition of ER stress may represent a potential therapeutic strategy to impede AKI-CKD transition.
Keywords: AKI-CKD transition; Apoptosis; Autophagy; ER stress; Fibrosis; Renal ischemia-reperfusion.
Copyright © 2018. Published by Elsevier B.V.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

