Microtubule nucleation by γ-tubulin complexes and beyond
- PMID: 30315097
- PMCID: PMC6281477
- DOI: 10.1042/EBC20180028
Microtubule nucleation by γ-tubulin complexes and beyond
Abstract
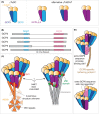
In this short review, we give an overview of microtubule nucleation within cells. It is nearly 30 years since the discovery of γ-tubulin, a member of the tubulin superfamily essential for proper microtubule nucleation in all eukaryotes. γ-tubulin associates with other proteins to form multiprotein γ-tubulin ring complexes (γ-TuRCs) that template and catalyse the otherwise kinetically unfavourable assembly of microtubule filaments. These filaments can be dynamic or stable and they perform diverse functions, such as chromosome separation during mitosis and intracellular transport in neurons. The field has come a long way in understanding γ-TuRC biology but several important and unanswered questions remain, and we are still far from understanding the regulation of microtubule nucleation in a multicellular context. Here, we review the current literature on γ-TuRC assembly, recruitment, and activation and discuss the potential importance of γ-TuRC heterogeneity, the role of non-γ-TuRC proteins in microtubule nucleation, and whether γ-TuRCs could serve as good drug targets for cancer therapy.
Keywords: MTOC; centrosome; g-TuRC; gamma-tubulin ring complex; microtubule.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



Similar articles
-
Drosophila melanogaster gamma-TuRC is dispensable for targeting gamma-tubulin to the centrosome and microtubule nucleation.J Cell Biol. 2006 Feb 13;172(4):517-28. doi: 10.1083/jcb.200511071. J Cell Biol. 2006. PMID: 16476773 Free PMC article.
-
GCP-WD mediates γ-TuRC recruitment and the geometry of microtubule nucleation in interphase arrays of Arabidopsis.Curr Biol. 2014 Nov 3;24(21):2548-55. doi: 10.1016/j.cub.2014.09.013. Epub 2014 Oct 16. Curr Biol. 2014. PMID: 25438942
-
Insights into the assembly and activation of the microtubule nucleator γ-TuRC.Nature. 2020 Feb;578(7795):467-471. doi: 10.1038/s41586-019-1896-6. Epub 2019 Dec 19. Nature. 2020. PMID: 31856152
-
Microtubule nucleation: The waltz between γ-tubulin ring complex and associated proteins.Curr Opin Cell Biol. 2021 Feb;68:124-131. doi: 10.1016/j.ceb.2020.10.004. Epub 2020 Nov 12. Curr Opin Cell Biol. 2021. PMID: 33190097 Review.
-
Regulation of microtubule nucleation mediated by γ-tubulin complexes.Protoplasma. 2017 May;254(3):1187-1199. doi: 10.1007/s00709-016-1070-z. Epub 2017 Jan 10. Protoplasma. 2017. PMID: 28074286 Review.
Cited by
-
MZT2A promotes NSCLC viability and invasion by increasing Akt phosphorylation via the MOZART2 domain.Cancer Sci. 2021 Jun;112(6):2210-2222. doi: 10.1111/cas.14900. Epub 2021 Apr 7. Cancer Sci. 2021. PMID: 33754417 Free PMC article.
-
Conformational Regulation of Vertebrate γ-Tubulin Ring Complexes by CM1 Proteins.Cytoskeleton (Hoboken). 2025 Aug;82(8):513-515. doi: 10.1002/cm.21979. Epub 2024 Dec 18. Cytoskeleton (Hoboken). 2025. PMID: 39692259 Free PMC article. No abstract available.
-
To nucleate or not, that is the question in neurons.Neurosci Lett. 2021 Apr 23;751:135806. doi: 10.1016/j.neulet.2021.135806. Epub 2021 Mar 8. Neurosci Lett. 2021. PMID: 33705928 Free PMC article. Review.
-
Dynamic Changes of Brain Cilia Transcriptomes across the Human Lifespan.Int J Mol Sci. 2021 Sep 27;22(19):10387. doi: 10.3390/ijms221910387. Int J Mol Sci. 2021. PMID: 34638726 Free PMC article.
-
Wdr47, Camsaps, and Katanin cooperate to generate ciliary central microtubules.Nat Commun. 2021 Oct 4;12(1):5796. doi: 10.1038/s41467-021-26058-5. Nat Commun. 2021. PMID: 34608154 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

