Electrostatic repulsion causes anticooperative DNA binding between tumor suppressor ETS transcription factors and JUN-FOS at composite DNA sites
- PMID: 30315111
- PMCID: PMC6290139
- DOI: 10.1074/jbc.RA118.003352
Electrostatic repulsion causes anticooperative DNA binding between tumor suppressor ETS transcription factors and JUN-FOS at composite DNA sites
Abstract
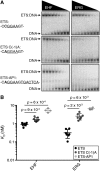
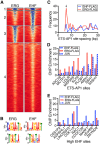
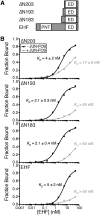
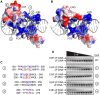
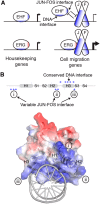
Many different transcription factors (TFs) regulate gene expression in a combinatorial fashion, often by binding in close proximity to each other on composite cis-regulatory DNA elements. Here, we investigated how ETS TFs bind with the AP1 TFs JUN-FOS at composite DNA-binding sites. DNA-binding ability with JUN-FOS correlated with the phenotype of ETS proteins in prostate cancer. We found that the oncogenic ETS-related gene (ERG) and ETS variant (ETV) 1/4/5 subfamilies co-occupy ETS-AP1 sites with JUN-FOS in vitro, whereas JUN-FOS robustly inhibited DNA binding by the tumor suppressors ETS homologous factor (EHF) and SAM pointed domain-containing ETS TF (SPDEF). EHF bound ETS-AP1 DNA with tighter affinity than ERG in the absence of JUN-FOS, possibly enabling EHF to compete with ERG and JUN-FOS for binding to ETS-AP1 sites. Genome-wide mapping of EHF- and ERG-binding sites in prostate epithelial cells revealed that EHF is preferentially excluded from closely spaced ETS-AP1 DNA sequences. Structural modeling and mutational analyses indicated that adjacent positively charged surfaces from EHF and JUN-FOS use electrostatic repulsion to disfavor simultaneous DNA binding. Conservation of positive residues on the JUN-FOS interface identified E74-like ETS TF 1 (ELF1) as an additional ETS TF exhibiting anticooperative DNA binding with JUN-FOS, and we found that ELF1 is frequently down-regulated in prostate cancer. In summary, divergent electrostatic features of ETS TFs at their JUN-FOS interface enable distinct binding events at ETS-AP1 DNA sites, which may drive specific targeting of ETS TFs to facilitate distinct transcriptional programs.
Keywords: AP1 transcription factor (AP1); ETS transcription factor family; JUN–FOS complex; gene expression; prostate cancer; protein–DNA interaction; protein–protein interaction; transcriptional regulation; tumor suppressor gene.
© 2018 Madison et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

