A Distinct Advantage to Intraarterial Delivery of 89Zr-Bevacizumab in PET Imaging of Mice With and Without Osmotic Opening of the Blood-Brain Barrier
- PMID: 30315146
- PMCID: PMC6495238
- DOI: 10.2967/jnumed.118.218792
A Distinct Advantage to Intraarterial Delivery of 89Zr-Bevacizumab in PET Imaging of Mice With and Without Osmotic Opening of the Blood-Brain Barrier
Abstract
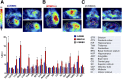
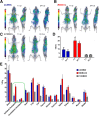
Glioblastoma multiforme (GBM) is the most aggressive and common type of brain cancer. Five-year survival rates are below 12%, even with the most aggressive trimodal therapies. Poor blood-brain barrier (BBB) permeability of therapeutics is a major obstacle to efficacy. Intravenous administration of bevacizumab is the standard treatment for GBM. It has been recently demonstrated that a single intraarterial infusion of bevacizumab provides superior therapeutic outcomes in patients with recurrent GBM. Further GBM treatment benefits can be achieved through opening of the BBB before intraarterial infusion of bevacizumab. However, a rationale for intraarterial delivery and BBB opening when delivering antibodies is lacking. A method facilitating quantification of intraarterial delivery of bevacizumab is needed for more effective and personalized GBM treatment. Here, we demonstrate such a method using PET imaging of radiolabeled bevacizumab. Methods: Bevacizumab was conjugated with deferoxamine and subsequently radiolabeled with 89Zr. 89Zr-bevacizumab deferoxamine (89Zr-BVDFO) was prepared with a specific radioactivity of 81.4 ± 7.4 MBq/mg (2.2 ± 0.2 μCi/mg). Brain uptake of 89Zr-BVDFO on carotid artery and tail vein infusion with an intact BBB or with BBB opening with mannitol was initially monitored by dynamic PET, followed by whole-body PET/CT at 1 and 24 h after infusion. Th ex vivo biodistribution of 89Zr-BVDFO was also determined. Results: Intraarterial administration of 89Zr-BVDFO resulted in gradual accumulation of radioactivity in the ipsilateral hemisphere, with 9.16 ± 2.13 percentage injected dose/cm3 at the end of infusion. There was negligible signal observed in the contralateral hemisphere. BBB opening with mannitol before intraarterial infusion of 89Zr-BVDFO resulted in faster and higher uptake in the ipsilateral hemisphere (23.58 ± 4.46 percentage injected dose/cm3) and negligible uptake in the contralateral hemisphere. In contrast, intravenous infusion of 89Zr-BVDFO and subsequent BBB opening did not lead to uptake of radiotracer in the brain. The ex vivo biodistribution results validated the PET/CT studies. Conclusion: Our findings demonstrate that intraarterial delivery of bevacizumab into the brain across an osmotically opened BBB is effective, in contrast to the intravenous route.
Keywords: brain; drug delivery; endovascular; molecular imaging; nuclear medicine.
© 2019 by the Society of Nuclear Medicine and Molecular Imaging.
Figures


References
-
- Grossman R, Burger P, Soudry E, et al. MGMT inactivation and clinical response in newly diagnosed GBM patients treated with Gliadel. J Clin Neurosci. 2015;22:1938–1942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
