Variants in exons 5 and 6 of ACTB cause syndromic thrombocytopenia
- PMID: 30315159
- PMCID: PMC6185941
- DOI: 10.1038/s41467-018-06713-0
Variants in exons 5 and 6 of ACTB cause syndromic thrombocytopenia
Erratum in
-
Author Correction: Variants in exons 5 and 6 of ACTB cause syndromic thrombocytopenia.Nat Commun. 2018 Nov 19;9(1):4930. doi: 10.1038/s41467-018-07404-6. Nat Commun. 2018. PMID: 30451859 Free PMC article.
Abstract
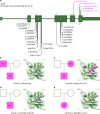
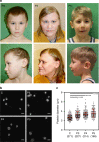
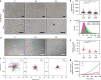
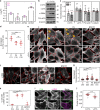
Germline mutations in the ubiquitously expressed ACTB, which encodes β-cytoplasmic actin (CYA), are almost exclusively associated with Baraitser-Winter Cerebrofrontofacial syndrome (BWCFF). Here, we report six patients with previously undescribed heterozygous variants clustered in the 3'-coding region of ACTB. Patients present with clinical features distinct from BWCFF, including mild developmental disability, microcephaly, and thrombocytopenia with platelet anisotropy. Using patient-derived fibroblasts, we demonstrate cohort specific changes to β-CYA filament populations, which include the enhanced recruitment of thrombocytopenia-associated actin binding proteins (ABPs). These perturbed interactions are supported by in silico modeling and are validated in disease-relevant thrombocytes. Co-examination of actin and microtubule cytoskeleton constituents in patient-derived megakaryocytes and thrombocytes indicates that these β-CYA mutations inhibit the final stages of platelet maturation by compromising microtubule organization. Our results define an ACTB-associated clinical syndrome with a distinct genotype-phenotype correlation and delineate molecular mechanisms underlying thrombocytopenia in this patient cohort.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- MA 1081/22-1/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- MA 1081/23-1/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- DI 2170/3-1/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- VWZN3012/Volkswagen Foundation/International
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

