Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies
- PMID: 30315224
- PMCID: PMC6755980
- DOI: 10.1038/s41380-018-0252-9
Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies
Abstract
Alterations in glutamatergic neurotransmission are implicated in the pathophysiology of depression, and the glutamatergic system represents a treatment target for depression. To summarize the nature of glutamatergic alterations in patients with depression, we conducted a meta-analysis of proton magnetic resonance (1H-MRS) spectroscopy studies examining levels of glutamate. We used the search terms: depress* AND (MRS OR "magnetic resonance spectroscopy"). The search was performed with MEDLINE, Embase, and PsycINFO. The inclusion criteria were 1H-MRS studies comparing levels of glutamate + glutamine (Glx), glutamate, or glutamine between patients with depression and healthy controls. Standardized mean differences (SMD) were calculated to assess group differences in the levels of glutamatergic neurometabolites. Forty-nine studies met the eligibility criteria, which included 1180 patients and 1066 healthy controls. There were significant decreases in Glx within the medial frontal cortex (SMD = -0.38; 95% CI, -0.69 to -0.07) in patients with depression compared with controls. Subanalyses revealed that there was a significant decrease in Glx in the medial frontal cortex in medicated patients with depression (SMD = -0.50; 95% CI, -0.80 to -0.20), but not in unmedicated patients (SMD = -0.27; 95% CI, -0.76 to 0.21) compared with controls. Overall, decreased levels of glutamatergic metabolites in the medial frontal cortex are linked with the pathophysiology of depression. These findings are in line with the hypothesis that depression may be associated with abnormal glutamatergic neurotransmission.
Conflict of interest statement
DMB receives research support from the Canadian Institutes of Health Research (CIHR), National Institutes of Health – US (NIH), Weston Brain Institute, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He received in-kind equipment support from Magventure for an investigator-initiated study. He received medication supplies for an investigator-initiated trial from Indivior. He has participated in an advisory board for Janssen.
MM has received grants and/or speaker’s honoraria from Asahi Kasei Pharma, Astellas Pharmaceutical, Daiichi Sankyo, Dainippon-Sumitomo Pharma, Eisai, Eli Lilly, Fuji Film RI Pharma, Janssen Pharmaceutical, Kracie, Meiji-Seika Pharma, Mochida Pharmaceutical, MSD, Novartis Pharma, Ono Yakuhin, Otsuka Pharmaceutical, Pfizer, Shionogi, Takeda Yakuhin, Tanabe Mitsubishi Pharma, and Yoshitomi Yakuhin within the past three years.
YN receives research grants from Japan Health Foundation, Meiji Yasuda Mental Health Foundation, Mitsui Life Social Welfare Foundation, Takeda Science Foundation, SENSHIN Medical Research Foundation, Health Science Center Foundation, and Daiichi Sankyo Scholarship Donation Program. He receives equipment-in-kind support for an investigator-initiated study from Magventure Inc.
ZJD has received within the last 3 years both research and equipment in-kind support for an investigator-initiated study through Brainsway Ltd. and Magventure.
EP has received funding from the Vanier Canada Graduate Scholarship, the Ontario Graduate Scholarship, and the Canada Graduate Scholarship—Master’s.
AG has received support from the United States National Institute of Health, CIHR, OMHF, Consejo Nacional de Ciencia y Tecnología, the Instituto de Ciencia y Tecnología del DF, the Brain & Behavior Research Foundation (Formerly NARSAD), the Ontario Ministry of Health and Long-Term Care, the Ontario Ministry of Research and Innovation Early Research Award, and Janssen.
NS has received fellowship grants from CIHR, research support from Japan Society for the Promotion of Science, Japan Research Foundation for Clinical Pharmacology, Naito Foundation, Uehara Memorial Foundation, Takeda Science Foundation, Daiichi Sankyo, and MSD, manuscript fees or speaker’s honoraria from Dainippon Sumitomo Pharma and Yoshitomi Yakuhin within the past three years. Other authors have no financial or other relationship relevant to the subject of this manuscript.
TK has received consultant fees from Dainippon Sumitomo, Novartis, Otsuka and speaker’s honoraria from Banyu, Eli Lilly, Dainippon Sumitomo, Janssen, Novartis, Otsuka and Pfizer. He has received grant support from the Pfizer Health Research, Takeda, Tanabe-Mitsubishi, Dainippon-Sumitomo, Otsuka and Mochida.
JHM have received operating grant funds for other studies from Janssen in the past 5 years. JHM has been a consultant to Mylan, Lundbeck, Takeda, Teva, and Trius in the past 5 years. JHM is an inventor on five patents of blood markers to predict brain inflammation or to diagnose affective disorders, and a dietary supplement to reduce depressed mood postpartum.
Figures
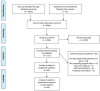


References
-
- Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185:1–10. - PubMed
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. - PubMed
-
- Tordera RM, Totterdell S, Wojcik SM, Brose N, Elizalde N, Lasheras B, et al. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1) Eur J Neurosci. 2007;25:281–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

