Different phenotypes of non-classical monocytes associated with systemic inflammation, endothelial alteration and hepatic compromise in patients with dengue
- PMID: 30315653
- PMCID: PMC6328998
- DOI: 10.1111/imm.13011
Different phenotypes of non-classical monocytes associated with systemic inflammation, endothelial alteration and hepatic compromise in patients with dengue
Abstract
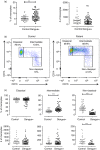
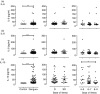
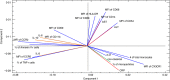
Although dengue can progress to severe stages, the exact causes of this phenomenon are unknown; however, the possibility of monocyte participation is acknowledged. It has been suggested that monocyte subsets (classical, intermediate and non-classical) play differential roles in dengue immunopathology. Therefore, we determined the count of monocyte subsets and obtained the clinical information of patients with dengue. We noted a significant decrease in the count of non-classical monocytes in patients compared with controls. With this finding, we focused on studying the phenotype of non-classical monocytes in the present study. An increase in activation and differentiation markers, such as CD64, CD86, the percentage of tumor necrosis factor-α+ cells and exposure of phosphatidylserine, were recorded in the non-classical monocytes of patients compared with controls. Moreover, a significant decrease in the expression of CX3CR1 with a corresponding increase in the expressions of CCR2, CCR5, CD11b and CD54 was detected in the non-classical monocytes of patients in comparison with that of the controls. Significant increases in the frequency of microparticles from endothelium and in the concentrations of interleukin-6 (IL-6), IL-8 and IL-10 were noted in the plasma of patients. These findings demonstrate that in patients with dengue, non-classical monocytes are activated, exhibiting a phenotype associated with more differentiation, produces tumor necrosis factor-α and has a profile of less endothelial surveillance closer to the cellular migration. These changes were associated with hepatic compromise, endothelial alteration and high concentration of circulating cytokines. Hence, alterations of non-classical monocytes seem to be associated with the immunopathology of dengue infection.
Keywords: dengue; monocyte subsets; monocytes; non-classical monocytes; severe dengue.
© 2018 John Wiley & Sons Ltd.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

