Eosinophilia and reduced STAT3 signaling affect neutrophil cell death in autosomal-dominant Hyper-IgE syndrome
- PMID: 30315710
- PMCID: PMC6587726
- DOI: 10.1002/eji.201847650
Eosinophilia and reduced STAT3 signaling affect neutrophil cell death in autosomal-dominant Hyper-IgE syndrome
Abstract
The autosomal-dominant hyper-IgE syndrome (HIES), caused by mutations in STAT3, is a rare primary immunodeficiency that predisposes to mucocutaneous candidiasis and staphylococcal skin and lung infections. This infection phenotype is suggestive of defects in neutrophils, but data on neutrophil functions in HIES are inconsistent. This study was undertaken to functionally characterize neutrophils in STAT3-deficient HIES patients and to analyze whether the patients` eosinophilia affects the neutrophil phenotype in S. aureus infection. Neutrophil functions and cell death kinetics were studied in eight STAT3-deficient patients. Moreover, the response of STAT3-deficient neutrophils to S. aureus and the impact of autologous eosinophils on pathogen-induced cell death were analyzed. No specific aberrations in neutrophil functions were detected within this cohort. However, the half-life of STAT3-deficient neutrophils ex vivo was reduced, which was partially attributable to the presence of eosinophils. Increased S. aureus-induced cell lysis, dependent on the staphylococcal virulence controlling accessory gene regulator (agr)-locus, was observed in STAT3-deficient neutrophils and upon addition of eosinophils. Accelerated neutrophil cell death kinetics may underlie the reported variability in neutrophil function testing in HIES. Increased S. aureus-induced lysis of STAT3-deficient neutrophils might affect pathogen control and contribute to tissue destruction during staphylococcal infections in HIES.
Keywords: HIES; S. aureus; STAT3; eosinophils; neutrophils.
© 2018 The Authors. European Journal of Immunology published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
None.
Figures
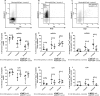
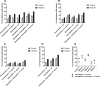

References
-
- Holland, S. M. , DeLeo, F. R. , Elloumi, H. Z. , Hsu, A. P. , Uzel, G. , Brodsky, N. , Freeman, A. F. et al., STAT3 mutations in the hyper‐IgE syndrome. N. Engl. J. Med. 2007. 357: 1608–1619. - PubMed
-
- Minegishi, Y. , Saito, M. , Tsuchiya, S. , Tsuge, I. , Takada, H. , Hara, T. , Kawamura, N. et al., Dominant‐negative mutations in the DNA‐binding domain of STAT3 cause hyper‐IgE syndrome. Nature 2007. 448: 1058–1062. - PubMed
-
- Freeman, A. F. , Kleiner, D. E. , Nadiminti, H. , Davis, J. , Quezado, M. , Anderson, V. , Puck, J. M. et al., Causes of death in hyper‐IgE syndrome. J. Allergy Clin. Immunol. 2007. 119: 1234–1240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

