Linking Lipid Metabolism to Chromatin Regulation in Aging
- PMID: 30316636
- PMCID: PMC6340780
- DOI: 10.1016/j.tcb.2018.09.004
Linking Lipid Metabolism to Chromatin Regulation in Aging
Abstract
The lifespan of an organism is strongly influenced by environmental factors (including diet) and by internal factors (notably reproductive status). Lipid metabolism is critical for adaptation to external conditions or reproduction. Interestingly, specific lipid profiles are associated with longevity, and increased uptake of certain lipids extends longevity in Caenorhabditis elegans and ameliorates disease phenotypes in humans. How lipids impact longevity, and how lipid metabolism is regulated during aging, is just beginning to be unraveled. This review describes recent advances in the regulation and role of lipids in longevity, focusing on the interaction between lipid metabolism and chromatin states in aging and age-related diseases.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
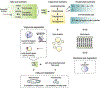

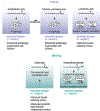

References
-
- Lawton KA et al. (2008) Analysis of the adult human plasma metabolome. ^ Pharmacogenomics 9, 383–97 - PubMed
-
- Moghadam NN et al. (2013) Age-induced perturbation in cell membrane phospholipid fatty acid profile of longevity-selected Drosophila melanogaster and corresponding control lines. Exp. Gerontol. 48, 1362–1368 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

